
Interaction between valproic acid and carbapenems: decreased plasma concentration of valproic acid and liver injury
Introduction
Valproic acid (VPA) is a broad-spectrum antiepileptic drug (AED) that is highly effective both in adults and children. It is one of the first-line drugs in the treatment of epilepsy (1). VPA is extensively metabolized by the liver; the main metabolic pathways of VPA include N-glucuronidation, mitochondrial b-oxidation, and cytochrome P-450 (CYP) microsomal oxidation (2). VPA has a complex and nonlinear pharmacokinetic profile and a target therapeutic range of 50 to 100 µg/mL (1). In China, perioperative seizure prophylaxis with AEDs in neurosurgery, especially VPA, has been advocated by Chinese experts and is widely used.
Carbapenems are a class of antibiotic agents with a wide spectrum of antimicrobial coverage (gram-positive, gram-negative, and anaerobic bacteria). The spread of extended-spectrum β-lactamase-caused infections and the proven efficacy of carbapenems against these organisms have led to its widespread use in practice. Meropenem has been shown to have a favourable safety profile, especially in central nervous system (CNS) tolerability. Therefore, for neurosurgical patients with active seizure disorder, or those who are at a high risk of developing seizures, meropenem is preferred when carbapenem antibiotics are indicated (3). However, a series of reports have indicated that the concomitant use of VPA and carbapenems, especially meropenem, can rapidly reduce the VPA plasma concentration (1,3-8). In our hospital, pharmacists found that the concomitant use of VPA and carbapenems was not completely avoided, and was accompanied decreased VPA concentration as well as a significantly higher alanine aminotransferase (ALT) value.
In this study, a retrospective analysis of VPA therapeutic drug monitoring (TDM) records and liver function monitoring records from neurosurgery inpatients was performed to address the following: (I) the effect of concomitant use of VPA and carbapenems on VPA levels; (II) whether the drug-drug interaction can influence the liver function of patients; and (III) whether the influence on liver function is different for different carbapenems. To our knowledge, there is no study that has investigated liver injury in cases of VPA and carbapenem co-administration.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-795).
Methods
Patients and data collection
In this retrospective analysis, the study population consisted of hospitalized patients from the Department of Neurosurgery at Shanxi Bethune Hospital from January 2018 to December 2019. This study was approved by the Shanxi Bethune Hospital Ethics Committee, and a waiver of informed consent was granted. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
The inclusion criteria were as follows: (I) patients aged 18–80 years, and weight >40 kg; (II) patients who received VPA monotherapy or concomitant therapy with meropenem or imipenem; (III) VPA, meropenem, and imipenem were administered via intravenous drip, and the daily dose of VPA was 1.2 mg/d; (IV) for patients who received VPA monotherapy, VPA TDM records were essential; whereas for patients who received concomitant therapy, VPA TDM records were nonessential. Prior to TDM, patients were treated with a constant VPA dose for at least five half-lives and attained a steady state of serum concentration; and (V) liver function testing records were required before and two weeks after administration of VPA monotherapy or concomitant therapy.
The exclusion criteria were as follows: (I) pregnant and breastfeeding women; (II) patients who used any known inducers or inhibitors of cytochrome p450 enzymes and UDP-glucuronosyltransferase during the study; (III) patients who used any known hepatotoxic drugs or liver protection drugs; (IV) patients receiving VPA irregularly; (V) patients with abnormal liver or renal function; and (VI) patients whose liver function testing records were missing or incomplete in the medical records.
A total of 141 inpatients satisfied the above criteria. The demographic characteristics, laboratory tests, prescription information, and VPA TDM data were obtained from electronic medical records (EMR) system.
Study design
Of the 141 inpatients, 75 patients with VPA TDM records were initially selected. Patients were divided into two groups based on whether they received VPA monotherapy or concomitant treatment with carbapenem antibiotics: a VPA monotherapy group and a VPA + carbapenem group. We compared the VPA levels between the aforementioned groups to evaluate the influence of carbapenem antibiotics on the VPA plasma concentration.
Secondly, all 141 patients were divided into three groups according to whether they received VPA monotherapy or concomitant treatment with meropenem or imipenem: a VPA monotherapy group, a VPA + meropenem group, and a VPA + imipenem group. We compared the liver injury rate of the above three groups to evaluate the influence of concomitant use of VPA with carbapenem antibiotics on liver function. Hepatotoxicity was defined as a new alanine aminotransferase (ALT) >3 times the upper limit of normal (ULN) (>120 U/L), alkaline phosphatase >2 times the ULN (>234 U/L), total bilirubin two times the ULN (>2 mg/dL), or doubling of the baseline value for ALT, alkaline phosphatase, or bilirubin if abnormal prior to initiation of valproate (9).
Statistical analysis
Continuous data were expressed as a mean ± SD for normally distributed data or as a median ± quartile range (QR) for non-normally distributed data. Categorical data were expressed as a number (%). Independent samples t-test was used for comparison of the VPA plasma concentration between the VPA monotherapy and VPA + carbapenem groups, and a paired t test was used to compare the ALT value before and after concomitant therapy. The chi-squared test was used to assess differences in the liver injury rate between the VPA monotherapy group, VPA + meropenem group, and VPA + imipenem group. All data were analyzed using SPSS version 22.0 (SPSS Inc. IBM Company), and P<0.05 was considered statistically significant.
Results
VPA plasma levels with and without carbapenem co-administration
Seventy-five patients with VPA TDM records were initially chosen from the 141 patients, with ages ranging from 25 to 70 years. Patients were most commonly admitted for cerebral hemorrhage (41, 54.6%), craniocerebral trauma (13, 17.3%), and aneurysm (12, 16.0%).
Patients were divided into two groups based on whether they received VPA therapy with or without carbapenem antibiotics: a VPA monotherapy group (N=66) and a VPA + carbapenem group (N=9). The demographics, type of carbapenem, and VPA serum concentrations of nine patients in the VPA + carbapenem group are shown in Table 1; eight cases exhibited subtherapeutic VPA levels only after few concomitant days, and one case reached therapeutic levels (50–100 µg/mL).

Full table
As shown in Table 2 and Figure 1, the VPA concentration of the VPA + carbapenem group (22.32±17.75 µg/mL) was much lower than that of the VPA monotherapy group (65.17±21.49 µg/mL) (P<0.01), suggesting that concomitant therapy with carbapenem could greatly reduce the VPA serum concentration.

Full table
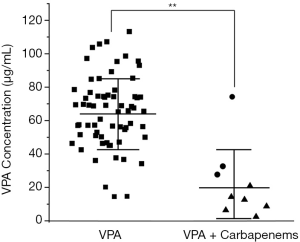
Liver injury rates of VPA therapy with and without carbapenem co-administration
The ages of all 141 included patients ranged from 19 to 80 years, and they were most commonly admitted for cerebral hemorrhage (80, 56.7%), craniocerebral trauma (28, 19.9%), and aneurysm (29, 20.6%).
Patients were divided into three groups based on whether they received VPA therapy with or without carbapenem antibiotics: a VPA monotherapy group (N=66), a VPA + imipenem group (N=27), and a VPA + meropenem group (N=48).
Table 3 displays the liver injury rate of patients who used VPA concomitantly with different types and doses of carbapenems. Since the dominating dose was 3.0 g in the meropenem or imipenem groups, the other doses were scattered and fewer in number. Thus, it was difficult to investigate the effects of different doses of carbapenem on liver function, and the different doses of meropenem or imipenem groups were respectively combined. As shown in Table 3, the liver injury rate was 3.70% and 35.42% in the imipenem and meropenem groups, respectively, and the liver injury rate of the VPA monotherapy group was 1.52%. The chi-squared test was used to compare the liver injury rate among three groups. The results showed that the liver injury rate of the three groups was significantly different (χ2=30.13, P<0.01, Table 4). Therefore, further pairwise comparisons were carried out according to the level of α'=0.0125. The results indicated that the liver injury rate of patients in the meropenem group (35.42%) was higher than that of patients in the imipenem (3.7%) or VPA monotherapy (1.52%) groups (P<0.01, Table 5). However, the difference between the imipenem group (3.7%) and VPA monotherapy group (1.52%) was not statistically significant (P>0.05).

Full table

Full table

Full table
The ALT values of the VPA + imipenem group were compared both before and after co-administration with imipenem. As shown in Table 6, the ALT values after co-administration (65.22±48.01 IU/L) were markedly higher than before (40.48±24.97 IU/L) (P<0.01).
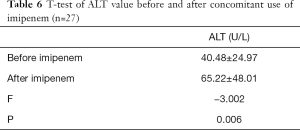
Full table
Discussion
VPA plasma concentrations with and without carbapenem co-administration
In this study, the plasma concentration of VPA in patients who received VPA with carbapenem was 22.32±21.77 µg/mL, which was significantly lower than that of patients who received VPA monotherapy (i.e., without carbapenem) (65.17±21.49 µg/mL). This indicates that carbapenem greatly reduced the serum concentration of VPA and increased risk of seizures. Nagai et al. was first to report that the serum concentration of VPA was decreased during the concomitant treatment with carbapenems, leading to epilepsy recurrence (10). The interaction between these medications has since been extensively reported, and the pharmacokinetic characteristics, as well as the clinical consequences of the interaction, have been further investigated (4,7,11,12). Some studies reported an increase in seizures during VPA-carbapenem combination therapy (5,13). Similar study shows comparable results in children who were treated with VPA for various seizures. All but two children in whom levels were monitored suffered a dramatic decline in levels when simultaneous treatment with carbapenems. Low plasma levels sometimes led to seizures (14).
The reduction of VPA serum concentration had the following characteristics. Firstly, the VPA plasma concentration decreased rapidly and could not be reversed by increasing the VPA dose. Carbapenem antibiotics may provide a new avenue for managing VPA overdose and toxicity. Dreucean et al. presented a case of utilization of meropenem to acutely lower VPA serum concentrations after an intentional overdose. In their study, the VPA level reduced from 278 to 193 µg/mL 3 h after intravenous administration of meropenem 1 g. Another 1 g dose of meropenem was given 8 h after the first dose, and the VPA levels reduced to 62 µg/mL approximately 1 h after the second dose (15).
Secondly, at least 7 days were required for the recovery of the VPA plasma concentration after discontinuation of carbapenem. Haroutiunian et al. found that the concentration of VPA significantly decreased within 24 hours (from 50.8±4.5 to 9.9±2.1 µg/mL) after continuous or intermittent use of meropenem. After discontinuation of meropenem, VPA plasma concentrations remained low for 7 days, and then gradually increased after 8–14 days, reaching values comparable to those before meropenem initiation. Different daily doses of VPA exhibited a similar pattern of decreased VPA concentrations (4).
Thirdly, all carbapenems demonstrated this interaction, however, the extent of VPA lowering appears to be lowest with imipenem (6,13). Wu et al. compared the interaction effect on VPA serum concentrations with different carbapenems. There was a significant decrease (P<0.001) in VPA concentrations with concomitant use of carbapenems: 72%±17%, 42%±22%, and 67%±19% in the ertapenem (N=9), imipenem/cilastatin (N=17), and meropenem (N=26) groups, respectively. The effect of ertapenem and meropenem on VPA was significantly more expressed than that of imipenem/cilastatin (P<0.005) (6). In present study, although there was no statistical comparison between the meropenem and imipenem groups (due to the limited samples), the VPA concentrations of six patients with meropenem were lower than those of three patients with imipenem.
The effect of concomitant use of VPA and carbapenem on liver function
In this study, there was a significant difference in the liver injury rates of the VPA + meropenem, VPA + imipenem, and VPA monotherapy groups. The liver injury rate of the VPA + meropenem group was 35.42%, which was higher than that of the VPA + imipenem group (3.7%) and VPA monotherapy group (1.52%). Although no significant differences were observed in the liver injury rate between the VPA + imipenem and VPA monotherapy groups, the ALT value of patients after co-administration with imipenem was markedly higher than before. Therefore, the data indicated that carbapenems might magnify the hepatotoxicity of VPA, and the effect of meropenem was significantly more expressed than that of imipenem.
The relationship between decreased VPA plasma concentration and liver injury
The interaction mechanism between VPA and carbapenem is complex, involving all aspects of absorption, distribution, metabolism, and excretion (1,16). The exact mechanism of the interaction has not yet been elucidated. The proposed mechanisms are as follows: inhibition of valproate glucuronide (VPA-G) hydrolysis (17), induction of valproate hepatic glucuronidation (17, 18), increased renal clearance of VPA-G (19,20), and increased distribution of VPA into red blood cells. At present, decreased VPA-G deglucuronidation due to inhibition of acylpeptide hydrolase (APEH) by carbapenem antibiotics may be the key mechanism in this drug–drug interaction (21-23).
Yamamura et al. performed experiments using rats to determine the site and mechanism of this drug interaction. In their study, panipenem treatment resulted in a significant reduction in biological half-life and a notable increase in total body clearance of VPA in normal and nephrectomized rats; meanwhile, hepatectomy abolished the interaction completely (19). Furthermore, Spriet et al. reported on a patient with severe liver impairment. However, they presented a unique situation in which VPA plasma concentrations were achieved and maintained within the therapeutic range of 50–100 mg/L, probably due to cirrhosis. The VPA concentrations were measured daily starting on day 8, and were 86, 79, 76, 77, 84, and 105 mg/L on days 8–13 during concomitant treatment with meropenem, and 100, 105, 99, 78, and 72 mg/L on days 14–18 after cessation of meropenem (24). Thus, the site of interaction was identified as the liver.
In our results, when VPA was concomitantly used with carbapenems, the VPA serum concentration was decreased, and at the same time, the liver function was impaired or the ALT value was increased. In addition, meropenem drug interaction analysis showed a higher reduction in VPA concentration compared with imipenem. Similarly, the hepatotoxicity of the meropenem drug interaction was significantly more expressed compared to imipenem. Our results revealed that mechanisms of the two effects caused by the concomitant use of VPA and carbapenem might be dependent.
This study had some limitations: it is a retrospective study leading to data loss, especially blood levels. the VPA concentrations of six patients with meropenem were lower than those of three patients with imipenem, but there was no statistical comparison of VPA blood levels due to the limited samples.
The interaction between VPA and carbapenems resulted in a decreased plasma concentration of VPA as well as possible liver injury. Clinicians should be aware of this potential interaction, and closely monitor VPA concentrations and liver function. Different carbapenems combined with VPA showed different effects on both VPA concentration and liver function, which revealed that the mechanisms of the two effects might be related.
Acknowledgments
Funding: This study was funded by the Health Commission of Shanxi Province, China (2021141).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-795
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-795
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-795). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Shanxi Bethune Hospital Ethics Committee, and a waiver of informed consent was granted.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Al-Quteimat O, Laila A. Valproate Interaction With Carbapenems: Review and Recommendations. Hosp Pharm 2020;55:181-7. [Crossref] [PubMed]
- Ketter TA, Frye MA, Cora-Locatelli G, et al. Metabolism and excretion of mood stabilizers and new anticonvulsants. Cell Mol Neurobiol 1999;19:511-32. [Crossref] [PubMed]
- Wen ZP, Fan SS, Du C, et al. Drug-drug interaction between valproic acid and meropenem: a retrospective analysis of electronic medical records from neurosurgery inpatients. J Clin Pharm Ther 2017;42:221-7. [Crossref] [PubMed]
- Haroutiunian S, Ratz Y, Rabinovich B, et al. Valproic acid plasma concentration decreases in a dose-independent manner following administration of meropenem: a retrospective study. J Clin Pharmacol 2009;49:1363-9. [Crossref] [PubMed]
- Miranda Herrero MC, Alcaraz Romero AJ, Escudero Vilaplana V, et al. Pharmacological interaction between valproic acid and carbapenem: what about levels in pediatrics? Eur J Paediatr Neurol 2015;19:155-61. [Crossref] [PubMed]
- Wu CC, Pai TY, Hsiao FY, et al. The effect of different carbapenem antibiotics (ertapenem, imipenem/cilastatin, and meropenem) on serum valproic acid concentrations. Ther Drug Monit 2016;38:587-92. [Crossref] [PubMed]
- Spriet I, Goyens J, Meersseman W, et al. Interaction between valproate and meropenem: a retrospective study. Ann Pharmacother 2007;41:1130-6. [Crossref] [PubMed]
- Vélez Díaz-Pallarés M, Delgado Silveira E, Alvarez Díaz AM, et al. Analysis of the valproic acid-meropenem interaction in hospitalised patients. Neurologia 2012;27:34-8. [Crossref] [PubMed]
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med 2006;354:731-9. [Crossref] [PubMed]
- Nagai K, Shimizu T, Togo A, et al. Decrease in serum levels of valproic acid during treatment with a new carbapenem, panipenem/betamipron. J Antimicrob Chemother 1997;39:295-6. [Crossref] [PubMed]
- Gu J, Huang Y. Effect of concomitant administration of meropenem and valproic acid in an elderly Chinese patient. Am J Geriatr Pharmacother 2009;7:26-33. [Crossref] [PubMed]
- Coves-Orts FJ, Borrás-Blasco J, Navarro-Ruiz A, et al. Acute seizures due to a probable interaction between valproic acid and meropenem. Ann Pharmacother 2005;39:533-7. [Crossref] [PubMed]
- Huang CR, Lin CH, Hsiao SC, et al. Drug interaction between valproic acid and carbapenems in patients with epileptic seizures. Kaohsiung J Med Sci 2017;33:130-6. [Crossref] [PubMed]
- Herrero M C M, Romero A J A, Vilaplana V E, et al. Pharmacological interaction between valproic acid and carbapenem: what about levels in pediatrics? Eur J Paediatr Neurol 2015;19:155-61. [Crossref] [PubMed]
- Dreucean D, Beres K, McNierney-Moore A, et al. Use of meropenem to treat valproic acid overdose. Am J Emerg Med 2019;37:2120.e5-7. [Crossref] [PubMed]
- Ishikawa T, Otaki H, Mizuta S, et al. Computational study of the competitive binding of valproic acid glucuronide and carbapenem antibiotics to acylpeptide hydrolase. Drug Metab Pharmacokinet 2017;32:201-7. [Crossref] [PubMed]
- Nakajima Y, Mizobuchi M, Nakamura M, et al. Mechanism of the drug interaction between valproic acid and carbapenem antibiotics in monkeys and rats. Drug Metab Dispos 2004;32:1383-91. [Crossref] [PubMed]
- Torii M, Takiguchi Y, Izumi M, et al. Carbapenem antibiotics inhibit valproic acid transport in Caco-2 cell monolayers. Int J Pharm 2002;233:253-6. [Crossref] [PubMed]
- Yamamura N, Imura K, Naganuma H, et al. Panipenem, a carbapenem antibiotic, enhances the glucuronidation of intravenously administered valproic acid in rats. Drug Metab Dispos 1999;27:724-30. [PubMed]
- Yokogawa K, Iwashita S, Kubota A, et al. Effect of meropenem on disposition kinetics of valproate and its metabolites inrabbits. Pharm Res 2001;18:1320-6. [Crossref] [PubMed]
- Suzuki E, Yamamura N, Ogura Y, et al. Identification of valproic acid glucuronide hydrolase as a key enzyme for the interaction of valproic acid with carbapenem antibiotics. Drug Metab Dispos 2010;38:1538-44. [Crossref] [PubMed]
- Suzuki E, Nakai D, Yamamura N, et al. Inhibition mechanism of carbapenem antibiotics on acylpeptide hydrolase, a key enzyme in the interaction with valproic acid. Xenobiotica 2011;41:958-63. [Crossref] [PubMed]
- Suzuki E, Nakai D, Ikenaga H, et al. In vivo inhibition of acylpeptide hydrolase by carbapenem antibiotics causes the decrease of plasma concentration of valproic acid in dogs. Xenobiotica 2016;46:126-31. [Crossref] [PubMed]
- Spriet I, Willems L. No interaction between valproate and meropenem in a cirrhotic patient. Ann Pharmacother 2011;45:1167-8. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


