Changes in clinical biochemical indexes of patients with heart failure with preserved ejection fraction or patients with hypertensive heart disease before and after treadmill exercise
Introduction
As the incidence of hypertension continues to rise in China, the prevalence of hypertensive heart disease (HHD) is increasing year by year (1). Early HHD is often accompanied by left ventricular hypertrophy and decreased diastolic function, which gradually causes decreased myocardial systolic function and increased pulmonary circulatory resistance, resulting in chest tightness and shortness of breath, and eventually leading to heart failure (HF) (2). However, some patients with HHD experience normal or slightly reduced cardiac output for a long time, which is referred to as HF with preserved ejection fraction (HFpEF) (3).
Clinical data from the Chinese Heart Failure Center show that HFpEF accounted for 41% of HF cases in 2019, and is gradually becoming a major type of HF, which poses a serious threat to life, carrying a 5-year case fatality rate of 50% (4,5). At present, HFpEF is mainly diagnosed based on the patient’s medical history, clinical symptoms, and comprehensive evaluation of cardiac ultrasound. However, due to some patients with HFpEF displaying a normal ejection fraction, the syndrome can be easily misdiagnosed, resulting in a delay to treatment (6). Consequently, active exploration of sensitive diagnostic indicators for HFpEF has become a hot topic in the cardiovascular research field.
Since its inception, the treadmill exercise test has been widely used to evaluate various heart diseases (7). Its mechanism is to stimulate the heart to doing extra work, through which potential cases can be identified. In the past, changes in heart rate or cardiogram indexes were commonly used as judgment indexes of treadmill exercise tests; however, few studies have focused on clinical serum biochemical index changes. Therefore, this study aimed to explore and compare the changes and significance of clinical biochemical indexes in patients with HFpEF and patients with HHD before and after treadmill exercise. We present the following article in accordance with the STARD reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1361).
Methods
General information
This study enrolled patients with HFpEF (78 cases) and patients with HHD (78 cases) who were treated in Beijing Chaoyang Hospital from February 2020 to February 2021. The HFpEF group comprised 32 males and 46 females, aged between 40 and 76 years old, with an average age of 61.84±8.52 years. The hypertension duration ranged from 5 to 12 years, with an average of 9.37±2.30 years. The HHD group included 36 males and 42 females, aged from 41 to 75 years old, with an average age of 62.32±8.35 years. The hypertension duration ranged between 5 and 11 years, with an average of 9.21±2.26 years. There was no significant difference between the two groups of patients in terms of general information such as age, sex, and history of hypertension (P>0.05). The study was approved by Beijing Chaoyang Hospital Affiliated to Capital Medical University (2020-527), and an informed consent form was signed by each patient or a family member on their behalf. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) patients in the HHD group were with a clear history of hypertension whose transcardiac ultrasound examination results met the standards for HHD: (II) patients in the HFpEF group were with electrocardiography and echocardiography examination results meeting the diagnostic criteria for HFpEF set out in the “Chinese Guidelines for the Diagnosis and Treatment of Heart Failure (2014 Edition)” (8), [i.e., typical symptoms and signs of HF, and normal or slightly reduced (≥50%) left ventricular ejection fraction (LVEF)], and meet at least one of the following criteria: (I) abnormal diastolic function; (II) left ventricular hypertrophy or (and) left atrium enlargement; (III) no heart valve disease; and (IV) complete clinical information. The exclusion criteria were: (I) patients with secondary hypertension; (II) patients with severe liver and kidney failure; (III) patients with autoimmune disease, or acute or chronic infection; (IV) patients with congenital heart disease or cardiomyopathy; (V) patients with malignant tumors or severe bleeding tendency.
Treadmill exercise test
The treadmill exercise test was performed on a T2100 treadmill (GE, USA). According to the Bruce exercise program, the exercise endpoint was the physical exhaustion that the patient could no longer adhere to the exercise or reach the extreme heart rate at their age. Before and after the exercise test, the clinical biochemical indexes of the patients were tested, and receiver operating characteristic (ROC) curves were drawn to analyze the best cutoff values for the clinical biochemical indexes. In this study, the positive standard was defined as the levels of N-segment pro-brain natriuretic peptide (NT-proBNP) and cardiac troponin I (cTnI) were ≥ the best cutoff values, and the positive rate of HFpEF before and after exercise was calculated.
Detection of clinical biochemical indicators
Venous blood (5 mL) was collected from each participant before and after treadmill exercise in a sodium citrate anticoagulation tube and left to stand for 1 hour. The blood samples were then centrifuged at 3,000 r/min for 10 minutes, and the supernatant was collected for further testing. Glycosylated hemoglobin A1C (HbA1c), low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C), and total cholesterol (TC) were detected before and after treadmill exercise using the DxC 800 automatic biochemical analyzer (Beckman, USA). The plasma levels of cTnI were detected by enzyme-linked immunosorbent assay (ELISA), and those of N-segment pro-brain natriuretic peptide (NT-proBNP) were detected by electrochemiluminescence.
Observation indicators
Clinical biochemical indexes including HbA1c, LDL-C, HDL-C, TC, NT-proBNP, and cTnI were determined and compared between the two groups before and after exercise. ROC curve analysis was performed to determine the best cutoff values for the clinical biochemical indexes for the diagnosis of HFpEF before treadmill exercise. The best ROC cutoff values were used as the positive judgment standard to compare the positive rates of the biochemical indexes in the diagnosis of HFpEF before and after exercise.
Statistical analysis
Data were analyzed using SPSS 21.0 statistical software (IBM, New York, USA). The measurement data were expressed as mean ± standard deviation (), and comparisons between the two groups were conducted by t-test. Paired sample t tests were used to analyze differences before and after exercise in the same group. Count data were expressed as rate (%), and analyzed using the chi-squared (χ2) test. ROC curves were drawn to analyze the best cutoff values of the clinical biochemical indexes in the diagnosis of HFpEF before treadmill exercise. Results with P<0.05 were considered to be statistically significant.
Results
Comparison of biochemical indexes before treadmill exercise
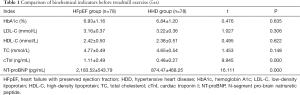
Before treadmill exercise, the levels of HbA1c, LDL-C, HDL-C, and TC displayed no statistically significant differences between the two groups (P>0.05); however, the NT-proBNP and cTnI levels were significantly higher in the HFpEF group than the HHD group (P<0.05, Table 1).
ROC curve analysis
The ROC curve analysis revealed that the areas under the curves (AUCs) of plasma NT-proBNP and cTnI for the diagnosis of HFpEF before treadmill exercise were 0.810 and 0.843, respectively, with the best cutoff values of plasma NT-proBNP and cTnI being 2,248.24 pg/mL and 1.14 ng/mL, respectively (Figure 1).

Comparison of biochemical indexes after treadmill exercise
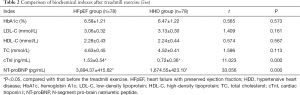
After treadmill exercise, the levels of HbA1c, LDL-C, HDL-C, and TC in the two groups were compared with those before treadmill exercise, and no statistically significant differences were found (P>0.05). However, the levels of NT-proBNP and cTnI in both groups after treadmill exercise were significantly higher than those before exercise, and the increase was more significant in the HFpEF group than the HHD group (P<0.05, Table 2).
Comparison of positive rates before and after treadmill exercise
The positive rates of plasma NT-proBNP and cTnI levels in the diagnosis of HFpEF after treadmill exercise were significantly higher than those before exercise (P<0.05, Table 3).

Full table
Discussion
With the aging of society, hypertension is increasing in incidence. If hypertension cannot be effectively controlled for a long time, it will induce changes in the structure and function of the heart, leading to HHD (9). As the final stage of progression to heart disease, HF occurs frequently in patients with HHD. The onset of HF is dangerous and can even cause death without effective treatment (10). Despite being one of the high-incidence types of HF, HFpEF currently lacks a specific treatment plan (11). Therefore, in clinical practice, a correct diagnosis should be made early and symptomatic treatment should be given in time to control or slow the deterioration of HF symptoms as much as possible. Therefore, the search for a fast and accurate differential diagnostic method is an important direction for current HFpEF research.
Treadmill exercise tests are a non-invasive, simple, and cheap examination method, and are widely used in the diagnosis of coronary heart disease (12). By increasing the body’s exercise load, the treadmill exercise test induces myocardial ischemia, which is directly reflected on the electrocardiogram. However, because some HFpEF patients with normal ejection fraction show similar symptoms to those with HHD, and the specificity and sensitivity of treadmill exercise tests are not high enough to distinguish HFpEF and HHD, it is necessary to identify other effective indicators to diagnose and differentiate HFpEF accurately.
This study showed that before treadmill exercise, the differences in HbA1c, LDL-C, HDL-C, and TC were not statistically significant between patients with HFpEF and HHD; however, the NT-proBNP and cTnI levels of the HFpEF group were significantly higher than those of the HHD group. Results of ROC curve analysis showed that the best cutoff values of plasma NT-proBNP and cTnI for diagnosing HFpEF before treadmill exercise were 2248.24 pg/mL and 1.14 ng/mL, respectively, suggesting that patients with abnormally high plasma NT-proBNP and cTnI levels at rest state may have HFpEF.
Under normal conditions, cTnI is mainly distributed in the cytoplasm of myocardial cells; however, it could be released into the blood by the stimulation of the imbalance of myocardial oxygen supply and oxygen consumption, or direct myocardial injury (13). NT-proBNP, an inactive peptide fragment secreted by stretching and stimulation of the body’s ventricular myocytes, can reflect heart function, and has been widely used in the diagnosis and prognostic evaluation of HF (14). Clinical studies have confirmed that once heart morphology and function show pathological changes, cTnI and NT-proBNP, as markers of cardiac injury, change significantly, and are negatively correlated with LVEF (15-17), which was fully verified by the results of the ROC curve analysis of this study.
The results of this study further showed that after treadmill exercise, the levels of HbA1c, LDL-C, HDL-C, and TC in the two groups were not statistically significantly different to those measured before treadmill exercise. However, the levels of NT-proBNP and cTnI in the two groups were higher after exercise than before exercise, with these increases being more significant in the HFpEF group. These results suggest that treadmill exercise can increase the expression of plasma NT-proBNP and cTnI levels.
Some scholars have reported that the plasma levels of NT-proBNP and cTnI increase significantly immediately after exercise (18,19), and the possible mechanisms are as follows. First, exercise increases the pressure of myocardial cells, thereby prompting myocardial cells to release large quantities of cardiomyocyte markers such as NT-proBNP and cTnI, which constitutes a physiological adaptation process of hemodynamic stimulation. Second, the increases in serum NT-proBNP and cTnI levels after exercise may have various contributing factors that result in “reversible damage” to the myocardium. Currently, many controversies still surround the abnormal increases observed in NT-proBNP and cTnI levels after exercise, but it is generally believed that in healthy people, increases in indexes following exercise are mostly the result of adaptation to accommodate the demands of heart function (20).
In this study, the plasma NT-proBNP and cTnI levels of patients with HFpEF increased more significantly than those of patients with HDD after treadmill exercise, which may be due to patients with HFpEF having a higher degree of myocardial damage than hypertensive patients. Therefore, it is necessary to reduce ischemia after exhaustive exercise. In patients with acute myocardial injury caused by perfusion, the levels of NT-proBNP and cTnI increase significantly to protect the myocardium. Further, we found that the positive rates of plasma NT-proBNP and cTnI in the diagnosis of HFpEF were higher after exercise than before exercise, which suggests that changes in plasma NT-proBNP and cTnI levels after treadmill exercise can be used for the diagnosis and differentiation of HFpEF and HHD. Exercise on a treadmill increases the body’s heart load and oxygen consumption, which further promotes the heart’s systolic or diastolic function on the basis of the original heart disease. At the same time, exhaustive exercise exacerbates acute myocardial damage, resulting in the abnormal increase of NT-proBNP and cTnI levels.
In conclusion, treadmill exercise is beneficial to the recovery of patients with HFpEF and hypertensive heart disease. More importantly, changes in plasma NT-proBNP and cTnI levels after the treadmill exercise test can be used as sensitive indexes for the assistant diagnosis and differentiation of HFpEF and HHD, with high clinical application value. However, due to the small sample size of this study, its conclusions require verification through large-sample and multicenter analysis in further prospective studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1361
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1361
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1361). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Beijing Chaoyang Hospital Affiliated to Capital Medical University (2020-527) and an informed consent form was signed by each patient or a family member on their behalf. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hao G, Wang X, Chen Z, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012-2015. Eur J Heart Fail 2019;21:1329-37. [Crossref] [PubMed]
- Messerli FH, Rimoldi SF, Wilhelm M. Heart Failure With Preserved Ejection Fraction: A Late Stage of Hypertensive Heart Disease. J Am Coll Cardiol 2017;70:2458. [Crossref] [PubMed]
- Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med 2016;375:1868-77. [Crossref] [PubMed]
- Zhang Y, Zhang J, Butler J, et al. Contemporary Epidemiology, Management, and Outcomes of Patients Hospitalized for Heart Failure in China: Results From the China Heart Failure (China-HF) Registry. J Card Fail 2017;23:868-875. [Crossref] [PubMed]
- Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591-602. [Crossref] [PubMed]
- Pfeffer MA, Shah AM, Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res 2019;124:1598-617. [Crossref] [PubMed]
- Weisser B. Exercise Testing in Sports Medicine. Dtsch Arztebl Int 2018;115:731-2. [PubMed]
- Chinese Society of Cardiology of Chinese Medical Association. Chinese guidelines for the diagnosis and treatment of heart failure 2014. Zhonghua Xin Xue Guan Bing Za Zhi 2014;42:98-122. [PubMed]
- Gazewood JD, Turner PL. Heart Failure with Preserved Ejection Fraction: Diagnosis and Management. Am Fam Physician 2017;96:582-8. [PubMed]
- Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol 2020;17:559-73. [Crossref] [PubMed]
- Stacey RB, Hundley WG. Integrating Measures of Myocardial Fibrosis in the Transition from Hypertensive Heart Disease to Heart Failure. Curr Hypertens Rep 2021;23:22. [Crossref] [PubMed]
- Fitzgerald BT, Logan JK, Weldon A, et al. The prognostic value of estimating stroke volume before and after exercise during treadmill stress echocardiography. Echocardiography 2020;37:1809-19. [Crossref] [PubMed]
- Myhre PL, O'Meara E, Claggett BL, et al. Cardiac Troponin I and Risk of Cardiac Events in Patients With Heart Failure and Preserved Ejection Fraction. Circ Heart Fail 2018;11:e005312 [Crossref] [PubMed]
- Salah K, Stienen S, Pinto YM, et al. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 2019;105:1182-9. [Crossref] [PubMed]
- Cunningham JW, Vaduganathan M, Claggett BL, et al. Effects of Sacubitril/Valsartan on N-Terminal Pro-B-Type Natriuretic Peptide in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2020;8:372-81. [Crossref] [PubMed]
- Thawabi M, Hawatmeh A, Studyvin S, et al. Cardiac troponin and outcome in decompensated heart failure with preserved ejection fraction. Cardiovasc Diagn Ther 2017;7:359-66. [Crossref] [PubMed]
- Zhang S, Jin R, Li B. Serum NT-proBNP and TUG1 as novel biomarkers for elderly hypertensive patients with heart failure with preserved ejection fraction. Exp Ther Med 2021;21:446. [Crossref] [PubMed]
- Iwanuk N, Nolte I, Wall L, et al. Effect of Pimobendan on NT-proBNP and c troponin I before and after a submaximal exercise test in dogs with preclinical mitral valve disease without cardiomegaly - a randomised, double-blinded trial. BMC Vet Res 2019;15:237. [Crossref] [PubMed]
- Christou GA, Pagourelias ED, Anifanti MA, et al. Exploring the determinants of the cardiac changes after ultra-long duration exercise: The echocardiographic Spartathlon study. Eur J Prev Cardiol 2020;27:1467-77. [Crossref] [PubMed]
- Donaldson JA, Wiles JD, Coleman DA, et al. Left Ventricular Function and Cardiac Biomarker Release-The Influence of Exercise Intensity, Duration and Mode: A Systematic Review and Meta-Analysis. Sports Med 2019;49:1275-89. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)