
Comparative efficacy and safety of four classical prescriptions for clearing damp-heat recommended by clinical guidelines in treating rheumatoid arthritis: a network meta-analysis
Introduction
Rheumatoid arthritis (RA) is a common autoimmune disease (1,2) characterized by chronic, symmetrical, erosive polyarthritis, which affects about 1% of people worldwide. The most apparent symptoms of RA include joint swelling, pain, tenderness, and morning stiffness. The pathological manifestations (3) are persistent synovitis of joints, systemic inflammation, and autoantibody-positivity. The main principle of treating RA (4) is to reduce inflammation and pain in the short-term and delay joint deformity and damage in the long-term. At present, first-line drugs include non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids (GCs), and second-line drugs include disease-modifying anti-rheumatic drugs (DMARDs). Among them, DMARDs are divided into 3 types, namely conventional synthetic DMARDs (csDMARDs), biological DMARDs (bDMARDs), and targeted synthetic DMARDs (tsDMARDs). Although the drugs mentioned above have been widely used to deal with RA, the vast economic costs and incurable nature of the condition have seriously increased the risk of psychological burden. Additionally, intolerance of toxic side effects, such as gastrointestinal reactions, liver and kidney damage, bone marrow suppression, osteoporosis, and so on, undermine patients’ compliance with medications, making the treatment of RA a serious global challenge (2,5-7). Therefore, some experts and academics have begun to investigate complementary and alternative therapies. Like the overall concept of treating RA under systemic biology (8), based on the theory of syndrome differentiation with multiple components in Chinese herbal formulae (CHF), Chinese herbal medicine anticipated to have considerable clinical efficacy in treating RA potentially.
In traditional Chinese medicine (TCM), RA is called Bi syndrome, and its clinical theory has been relatively mature for a long time. Chinese herbal medicine has been used extensively to improve the quality of life of RA patients. Based on the principle of syndrome differentiation and treatment, some systematic reviews of the evidence (9-11) have manifested that CHF combined with csDMARDs for RA patients have certain synergistic effects in reducing ARs and improving efficacy, and so on. Particularly in recent years, considering the high correlation between clinically active RA and damp-heat arthralgia in the field of TCM (8), many studies have started to explore the clinical effects of various classical prescriptions for clearing heat and dampness. Some studies (12-15) have shown that if the TCM syndrome differentiation is damp-heat arthralgia syndrome, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are significantly higher in other syndrome types, and RA disease activity is the highest. For example, recent randomized controlled trials (RCTs) (16-18) have also demonstrated that the CHF for clearing dampness and heat, such as Simiao pill (SMP), Baihuguizhi decoction (BHGZD), Xuanbi decoction (XBD), combined with the csDMARD, can effectively improve RA symptoms, including reducing inflammatory reaction substances, and relieving joint pain and swelling. Concurrently, the safety of TCM has also been an issue of concern in China and internationally. Some anti-rheumatic monomers of herbs, such as tripterygium wilfordii, total glucosides of peony, and sinomenine (19-21) have been reported to damage despite having shown certain curative effects on the liver and kidney, reproductive system, and digestive tract. However, encouragingly, according to the data of the 2016–2020 National Adverse Drug Reaction Monitoring Center, among drugs with clinical ARs, the probability of ARs of herbs that clear heat and dampness have been low in all categories of TCM, with the incidence of adverse events (AEs) of 4–6% (Figure S1). We suppose that CHF of clearing damp-heat is normally safe and commonly used, which shows a certain clinical value.
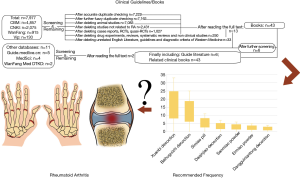
In RA treatment, there are many kinds of prescriptions for clearing damp-heat. By searching the clinical guidelines and books related to RA or Bi syndrome of the damp-heat type syndrome, we found that some formulae are more frequently recommended, including BHGZD, Dangguiniantong decoction (DGNTD), SMP, XBD, Daqinjiao decotion (DQJD), Ermiao san (EMS) and Sanmiao san (SMS). The retrieval process, detailed information of included guidelines and books, and recommended frequency of CHF can be seen in Table S1 and Figure 1. Nevertheless, the differences in efficacy and safety between different prescriptions are not clear. It is difficult for clinicians to choose the appropriate prescription for RA quickly. Therefore, we used a network meta-analysis (NMA) (22) method that can estimate direct and indirect comparison results to calculate the curative effect ranking and evaluate the differences in efficacy and safety of the above classical prescriptions combined with 1 kind of csDMARD in the treatment of RA. Simultaneously, to reduce the false-positive results caused by clinical differences between the CHF, we included RCTs in the study. Interventions need to be a certain CHF recommended by the guidelines/professional books to be eligible for inclusion. Additionally, the included population had to have damp-heat syndrome RA, and the composition of the decoction used did not involve the toxic herbs listed in Table S2 wherever possible. Moreover, to balance the confounding clinical heterogeneity factors between different treatment groups, we added trial sequential analysis (TSA) in this study to expand the sample statistics to verify the results. We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/apm-21-445). The protocol of this NMA was also registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020200116).
Methods
Search strategies
First, the relevant subject literature should be consulted, and the corresponding search words were initially formulated. By consulting experts in rheumatism and evidence-based medicine, the corresponding theme words and free words were supplemented, and the pre-retrieval protocol was submitted on the PROSPERO platform. After the expert group evaluated the retrieval results, the final retrieval strategy was further improved to reduce differences in study retrieval. A total of 7 electronic databases for systematic review searches were used, as follows: PubMed (https://www.ncbi.nlm.nih.gov/pubmed/); Cochrane Library (http://www.cochranelibrary.com/); EMBASE (http://www.embase.com/); SinoMed (CBM, http://www.sinomed.ac.cn/); China National Knowledge Infrastructure (CNKI, http://www.cnki.net/), China Science and Technology Journal Database (CQVIP, http://www.cqvip.com/), and Wanfang Data Knowledge Service Platform (http://www.wanfangdata.com.cn/index.html). There was no language restriction in the search strategy. Full-text retrieval was from the establishment of the databases to July 2020. Search strategies included the subject terms and free-text terms, which are displayed in Table S3. Two researchers independently searched, and any differences were resolved after discussion with a third researcher.
Inclusion criteria
Types of studies
All included studies were RCTs, and each trial evaluated the efficacy of CHF recommended by the guidelines combined with 1 csDMARD in the treatment of RA.
Participants
Participants were diagnosed with RA according to the accepted diagnostic criterion or clinical symptoms and signs. We used the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria as an inclusion criterion for diagnosis.
Interventions and comparators
The treatment group used the recommended CHF combined with 1 csDMARD (such as methotrexate or leflunomide). According to the guidelines and clinical books, the very frequently recommended CHF for clearing damp-heat with BHGZD, DGNTD, SMP, XBD, and DQJD. Based on the original prescription, modified decoctions were also included. The formulas EMS and SMS were not searched separately because SMP is a modified decoction of them. There were no restrictions on the quantity, dose, and dosage form of ingredients included in the prescription. The medication must have been consumed orally. The treatment cycle was at least 2 weeks or 3 courses. In the same study, basic drugs were the same among groups and considered as blank interventions. These drugs included low-dose hormones, NSAIDs, stomach medicine, folic acid, and so on.
Main and additional outcomes
Studies that reported 1 or more of the following pre-specified main or additional outcomes were included in this NMA. The primary outcomes included effective rate (ER), ACR 20/50/70, disease activity score-28 (DAS-28), AEs, adverse reactions (ARs), and serological examination, such as ESR, CRP, rheumatoid factors (RF), tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1). The secondary outcomes were signs and symptoms assessment, including swollen joint count (SJC), tender joint count (TJC), and morning stiffness time (MST).
Data extraction and quality assessment
First, we used Endnote X9 software (Clarivate Analytics, Philadelphia, PA, USA) to classify and sort the initially retrieved studies to exclude duplicate articles; second, according to the inclusion and exclusion criteria, the 2 researchers eliminated unqualified studies through the titles, keywords, and abstracts, filed the excluded literature, and recorded the reasons and quantity of the exclusion. For incomplete information reports, the original author was contacted to supplement relevant materials, and if that were not possible, it would be removed. Third, the 2 researchers used Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, USA) tables to extract data from the corresponding studies, including qualitative and quantitative data. All relevant information was independently and carefully screened by 2 reviewers, with any disputes between the 2 parties being, resolved by the third assessor. The datum extracted were as shown below: (I) baseline of variables including first author, year of publication, trial design method, a sample size of participants, age range, course of the disease, diagnostic criteria, intervention measures, duration of treatment, outcomes, and follow-up; (II) dichotomous data or continuous data of the pre-specified outcomes.
The 2 reviewers used the Cochrane risk bias assessment tool to evaluate the quality of the included studies, and the third reviewer cooperated to reach an agreement through discussion. The related items of bias risk tool recommended by the Cochrane Collaboration (23) provided the following 6 evaluation criteria, which were expressed in 3 states: high risk, low risk, and unclear. The 6 items included: (I) whether randomization was mentioned and its random methods were reasonable; (II) whether allocation concealment was mentioned and whether the method was effective; (III) whether the blind method was mentioned and whether it was correct; (IV) whether the outcome data were complete; (V) whether there was a suspicion of selective reporting; (VI) other sources of bias.
Strategy for data synthesis
We conducted a pairwise meta-analysis in a random-effects model by RevMan 5.3 software (Copenhagen: the Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and performed a Bayesian NMA using the Markov Chain Monte Carlo (MCMC) methods by Stata 14.0, R 4.0.2 (College Station, TX, USA), GeMTC 0.14.3 (http://drugis.org/software/addis1/getmc), and JAGS 4.3.0 software (http://mcmc-jags.sourceforge.net/). We calculated the binary data, such as the ER, ACR20/50/70, and so on, as the odds ratio (OR) with its 95% confidence interval (CI). For the continuous data, such as changes in physical function or inflammation markers, values were presented by mean differences (MD) with 95% CI. When the measurement standard was different, or the result variables were highly inconsistent between studies, standardized mean difference (SMD) was used (24,25).
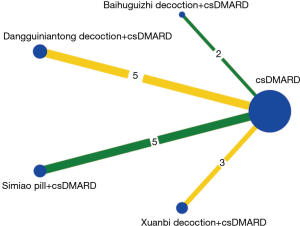
Each direct comparison of the interventions was drawn in Stata 14.0 software with a network plot command. Different lines had corresponding colors. Green, yellow, and red represented the risk of bias of the random method as low, unclear, and high, respectively. The node size was on behalf of the total sample size under 1 kind of intervention. If there had been a closed loop in the included interventions, an inconsistency test would have been performed; however, we found no direct evidence to form a closed loop, so the consistency test in direct and indirect comparison was unnecessary (26). Nevertheless, the research should also be in accord with the hypothesis of heterogeneity and consistency. There were many outcomes included in this article. To control the conclusions’ reliability, we only conducted the NMA for the outcomes, including more than 10 studies. A total of 20,000 iterations were set in the whole calculation process. To eliminate the influence of the original iteration, we set up the first burn-in of 5,000 iterations. The effects of each intervention in RA treatment were estimated, ranked, and clustered by the surface under the cumulative ranking curve (SUCRA) (22,27). These results might ultimately provide clinicians with treatment options via single or multiple factors.
Homogeneity hypothesis and publication bias
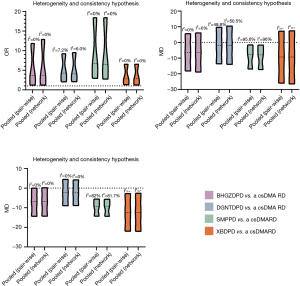
The homogeneity test of the NMA came from the direct comparison of the heterogeneity of the pairwise meta-analysis. The Q test was used to test the heterogeneity among the results of the included studies, and the test level was α=0.10. If the heterogeneity test result showed P>0.1, I2≤50%, it meant that the heterogeneity was low, and the fixed effects model was used for the combined analysis of the meta-analysis (28). Otherwise, the random effects model was used for the combined analysis of efficacy, and the source of the heterogeneity was analyzed. If there was significant heterogeneity in different studies, we performed subgroup analysis to address the source of heterogeneity. Considering the different CHF in the treatment of RA in this study, we thought that the prescription rule of the decoction and the different durations of disease were the 2 major factors of heterogeneity. Using these 2 factors, taking the ER, CRP, and ESR as examples, we compared the combined effect of different formulas in RA patients with that of sub-combination, observed an overlapping effect between groups, and then judged the significance of grouping. Sensitivity analysis by excluding 1 study after another in RevMan 5.3 software was performed to observe the impact of a study on the overall results. If outcomes involved more than 10 studies, a funnel plot was drawn to observe publication bias. Besides, the Egger’s regression (29) at significance P<0.05 quantitatively identified potential publication bias. Trim and fill method (30) was used to correct the estimates. Brooks Gelman Rubin’s diagnostic method was used to judge the goodness of fit in the stability of the results (31).
TSA
TSA was used for sample size estimation in meta-analysis. In a study, the required sample size was evaluated to the greatest extent. This could avoid the possibility of false positives in meta-analysis and avoid the waste of the resources brought about by multiple repeated tests. Required information size (RIS) was the sample size required for the estimation test. By observing whether the cumulative Z curve crossed the traditional boundary value, TSA boundary value, and RIS line, we judged whether the analysis result was statistically significant, whether there was a definite conclusion, and whether the sample size was up to the expected value.
Network pharmacology analysis
Concerning the ninth edition of the TCM internal medicine standard, we collected the composition of this 4 CHF. Through the TCM systems pharmacology (TCMSP) database (http://tcmspw.com/tcmsp.php), we searched for the related components of CHF following oral absorbability (OB) ≥30% and drug-likeness (DL) ≥0.18. The compositions of Talc, Gypsum, and Oryza sativa were determined using the TCM integrated database (TCMID, http://www.megabionet.org/tcmid/) and PharmMapper platform (http://www.lilab-ecust.cn/pharmmapper/) as supplements.
Secondly, the target proteins of compounds obtained from the TCMSP database were passed through the UniProt database (https://uniprot.org/) to be standardized. The Genecards database (https://www.genecards.org/) and the Online Mendelian Inheritance in Man (OMIM) database (https://omim.org/) were used to search the corresponding disease targets. Then, we selected humans as the search species on the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) platform (http://string-db.org/cgi/input.pl) and summed up the Venn results of the above targets. With 0.9 as the highest reliability, the core targets were selected. Finally, the results were imported into the software of Cytoscape 3.7.1 (https://cytoscape.org) to build a protein interaction network. The software R 4.0.2 software (https://www.R-project.org/) was used to analyze the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment.
Results
Retrieval results and baseline
We identified 2,180 articles in 7 databases. After deleting duplicate articles, 15 qualified studies were finally screened out according to the inclusion and exclusion criteria. The screening process is shown in Figure 2. A total of 15 RCTs investigated the efficacy of different decoctions combined with 1 csDMARD. The groups included BHGZD + csDMARD (BHGZDPD, 2 RCTs), DGNTD + csDMARD (DGNTDPD, 5 RCTs), SMP + csDMARD (SMPPD, 5 RCTs) and XBD + csDMARD (XBDPD, 3 RCTs). The related research of DQJD failed to meet the screening criteria, so it was not discussed in this NMA. The primary details are shown in Table 1.
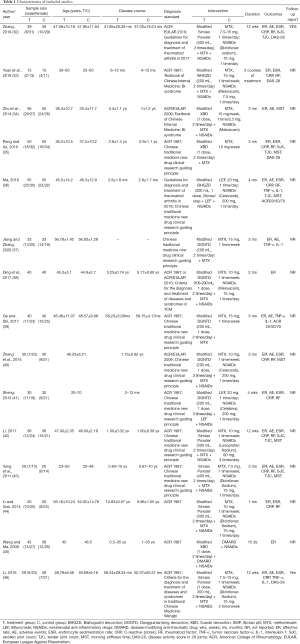
Full table
The included studies were all published in Chinese from 2008 to 2020, and the patients’ course of disease ranged from 2 months to 35 years. The components of CHF are shown in Table 2.
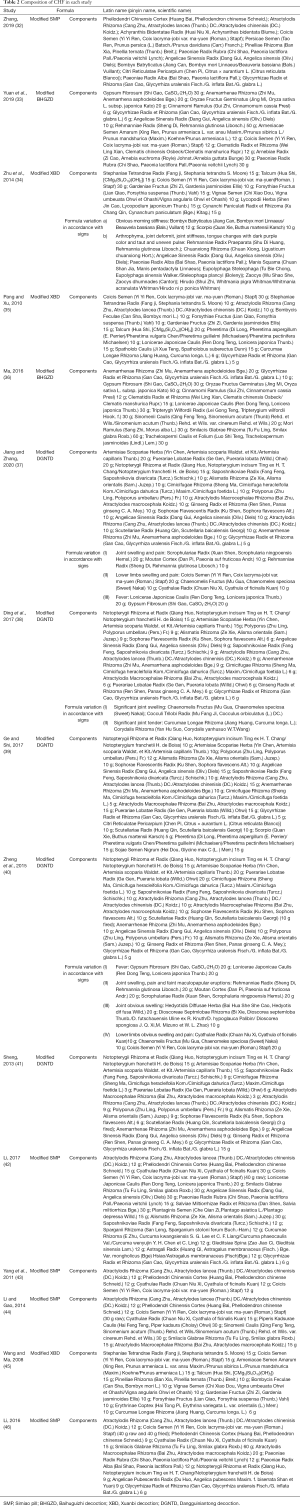
Full table
Quality assessment of included research
All included studies mentioned randomization, but only 7 trials (32,33,36,39,41,43,46) reported appropriate randomization methods; no studies explicitly reported allocation blinding; 1 study (41) mentioned blinding and related methods, and the rest did not report in detail; 5 RCTs (33,35,38,44,45) did not report any ARs or AEs, which might show the risk of selective reporting. The risk of bias plot is displayed in the Figure 3.
Evaluation of therapeutic efficacy
A pairwise meta-analysis and network meta-analysis
The results of ER
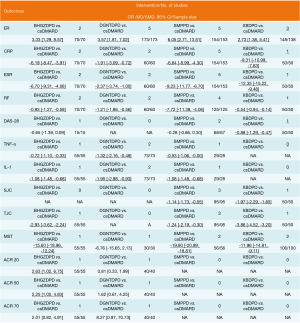
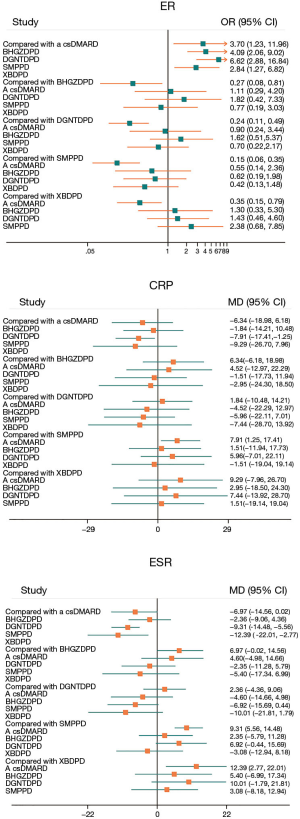
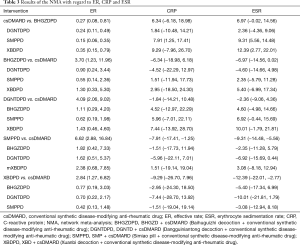
Including 1,079 participants, 15 studies reported the clinical efficiency of 4 CHF, each combined with 1 csDMARD, and compared it to the efficiency of csDMARD used alone. With a random-effects model set, the pairwise meta-analysis indicated that all 4 CHF + csDMARD had better treatment effects compared to the csDMARD used alone (BHGZD: OR =3.33, 95% CI: 1.29 to 8.57, I2=0%; DGNTD: OR =3.57, 95% CI: 1.81 to 7.02, I2=0%; SMP: OR =6.05, 95% CI: 2.71 to 13.51, I2=0% and XBD: OR =2.73, 95% CI: 1.38 to 5.41, I2=0%) (Figure 4, Table S4). A direct comparison of all interventions formed a network diagram (Figure 5). The results of NMA also showed that the 4 treatment groups had statistically significant differences concerning ER in CHF + csDMARD vs. csDMARD (BHGZD: OR =3.70, 95% CI: 1.23 to 11.96; DGNTD: OR =4.09, 95% CI: 2.06 to 9.02; SMP: OR =6.62, 95% CI: 2.88 to 16.84; XBD: OR =2.84, 95% CI: 1.27 to 6.82) (Figure 6, Table 3). Heterogeneity analysis in the pairwise meta-analysis showed that the homogeneity among the studies was acceptable. Comparing the indirect comparison and direct comparison results, the statistics of the models were stable (Figure 7).
Full table
The results of serological tests (CRP, ESR, RF, TNF-α and IL-1)
Regarding serological examination, by conducting the pairwise meta-analyses, CRP, ESR, and RF changes were observed in the 4 decoctions, and TNF-α and IL-1 were estimated in the BHGZD, DGNTD, and SMP treatment groups. There were statistically significant changes in CRP, ESR, and RF for the treatments of the 4 CHF (P<0.05). Compared with the csDMARD alone, BHGZDPD, DGNTDPD, and SMPPD could also reduce the levels of TNF-α and IL-1 inflammation (P<0.05) (Figure 4, Table S4). However, heterogeneity analysis showed that the SMP group had high heterogeneity in the CRP and ESR results (I2=90%, I2=80%). We performed subgroup analysis and found that the combined value between the groups had an interaction effect, and the P value was greater than 0.05, which showed that the different prescriptions and the patients’ course of disease had no obvious clinical heterogeneity in the study (Figure 8). We ran NMA with a random effects model. In terms of NMA, because some studies failed to report enough outcomes, we only carried it out for studies that involved all 4 interventions and contained more than 10 studies, to reduce the bias report due to the small sample size. Regarding changes in CRP and ESR levels, a total of 10 studies involving 667 patients were evaluated in NMA. Compared with the csDMARD alone, the results showed that SMPPD had a significant difference in efficacy. It showed a better effect on reducing CRP levels (MD =−7.91, 95% CI: −17.41 to −1.25). Moreover, significant differences were observed in ESR for XBDPD and SMPPD (MD =−12.39, 95% CI: −22.01 to −2.77; MD =−9.31, 95% CI: −14.48 to −5.56, respectively) (Figure 6,Table 3). The results of the pairwise meta-analysis and NMA were comparatively accordant, and the model was more reliable (Figure 7).

The results of signs and symptoms (TJC, SJC and MST)
Because the sample size was small, and there were only 3 interventions for some of the outcomes, we performed a pairwise meta-analysis instead of NMA. Therefore, the random-effects model was used for direct comparison. Compared with a csDMARD alone, 6 trials evaluated the efficacy of CHF combined with the csDMARD separately in the remission of morning stiffness for RA. With the exception of DGNTD, 3 herbal formulas showed a certain effect on alleviating morning stiffness (BHGZD: MD =−15.60, 95% CI: −18.96 to −12.24; SMPPD: MD =−19.90, 95% CI: −20.99 to −18.81; XBDPD: MD =−11.96, 95% CI: −14.81 to −9.11, P<0.05). A total of 5 studies integrated the effects of BHGZDPD, SMPPD, and XBDPD in treating joint tenderness of RA. Compared with one csDMARD, the combined group showed a certain therapeutic advantage in relieving aching joints (P<0.05). In terms of SJC, SMPPD and XBDPD appeared to be more effective than the csDMARD for relieving joint swelling (P<0.05) (Figure 4, Table S4).
The results of disease activity (DAS-28 and ACR 20 50 70)
In evaluating disease activity, the scores of ACR 20, ACR 50, ACR 70, and DAS-28 were more internationally recognized for use in RA patients. However, some studies did not report these 2 kinds of outcomes or did not specify the scoring criteria. Therefore, we just conducted pairwise meta-analyses with the random-effects model to evaluate these outcomes. The DAS-28 was determined in 5 studies. In DAS-28, there was no significant change in BHGZDPD or SMPPD compared with the csDMARD alone. The pairwise meta-analysis demonstrated that only XBDPD was superior to a csDMARD (SMD =−0.88, 95% CI: −1.29 to −0.47). A total of 2 trials evaluated values on the changes of ACR 20, 50, and 70. The evaluation of ACR 20 and ACR 50, it indicated that BHGZDPD had statistical significance in treating RA (MD =2.63, 95% CI: 1.02 to 6.75, MD =2.25, 95% CI: 1.05 to 4.83). There was no significant difference in other direct comparison results (Figure 4, Table S4).
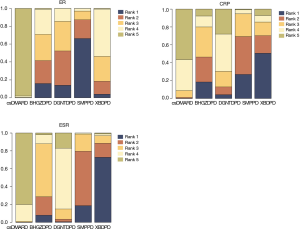
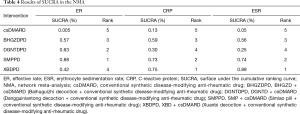
Ranking results and cluster analysis
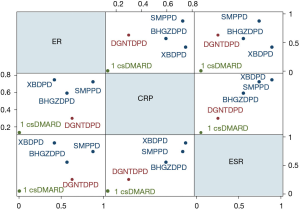
By different color occupancy areas, the ranking probabilities of different interventions regarding each outcome are presented in (Figure 9). From Rank 1 to Rank 4, color occupancy indicated the probability of superiority to inferiority. The larger the area occupied in Rank 1, the better the curative effect. In light of the calculation method shown in the study, the SUCRA value was calculated according to the rank probability. The SUCRA values in each NMA outcome are shown in Table 4. Higher values of the SUCRA recommended more advantageous therapies. The comprehensive rank results indicated that SMPPD might be the potential optimizing therapy. However, in clinical practice, when choosing treatment measures, several outcomes usually require comprehensive consideration. Cluster analysis showed that SMPPD, XBDPD, and BHGZDPD could have a certain synergy in terms of improving efficacy and reducing inflammation (Figure 10).
Full table
Safety
Of the 15 RCTs included, 5 trials (33.33%) did not record AEs/ARs during the treatment period, and the remaining 10 studies (66.67%) reported AEs/ARs. According to the treatment of different interventions, different types of AEs/ARs were reported (Table 5). The ARs mainly included abnormal liver function, abnormal renal function, blood test abnormalities, gastrointestinal reactions, cough, skin rash, myelosuppression. We could also draw 3 main findings: first, the incidences of ARs in patients who used a csDMARD alone (27.42%) or XBDPD (26.79%) were higher; second, SMPPD (6.45%) and DGNTDPD (6.77%) had lower incidences of ARs; third, the incidence of gastrointestinal reactions (10.84%) was the highest among all treatments.

Full table
Heterogeneity, publication bias and Brooks-Gelman-Rubin diagnosis
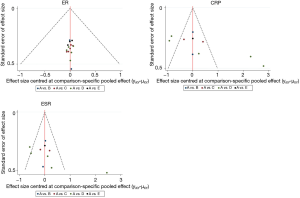
The studies shown in the funnel plot were close to the bottom or scattered, indicating small sample size bias and other potential biases (Figure 11). Egger’s test reported a publication bias in CRP results (P=0.033). Nevertheless, the correction using the trim and fill method for this bias did not alter the overall result, which meant that the pooled value was retained as reliable (Table 6 and Table S5). In CRP and ESR results, the SMPPD studies were scattered outside the interval, indicating that it might have been the source of heterogeneity between studies. To observe the source of heterogeneity, we also conducted a sensitivity analysis by removing each trial 1 by 1, and performed a subgroup analysis to find the confounding factors. It showed that the results of the pairwise meta-analysis were credible (Table S4). Heterogeneity may have come from the variation of intrinsic authenticity in the research of detection methods, but this needs to be further verified. After 5,000 iterations, the potential scale reduced factor (PSRF) was calculated. The median value and 97.5% of the reduction factor quickly reached stability, indicating that the model had stable convergence and reliable statistical results (Figure S2). However, due to the small sample size, the results might have exaggerated the therapeutic effect, so we should treat the conclusions with caution.

Full table
Sample cumulative results
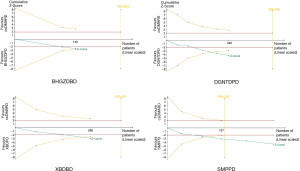
The TSA results showed that the cumulative Z-value curve of XBDPD and DGNTDPD exceeded the traditional curve and crossed the TSA threshold, indicating that a positive conclusion was obtained before the expected amount of information was reached. The SMPPD results showed that the cumulative Z-value curve exceeded the traditional curve, and crossed the TSA threshold. Simultaneously, the cumulative amount of information reached the expected amount, indicating that a positive conclusion had been obtained, and this conclusion would not change with the increase of sample size. However, in the BHGZDPD group, the cumulative Z-value curve exceeded the traditional curve. However, it did not cross the TSA threshold, and the cumulative amount of information did not reach the expected amount, indicating that the meta-analysis might have had a false-positive conclusion, and more trials are needed to be included to confirm the efficacy (Figure 12).
Results of network pharmacology analysis
Composition information of 4 CHF
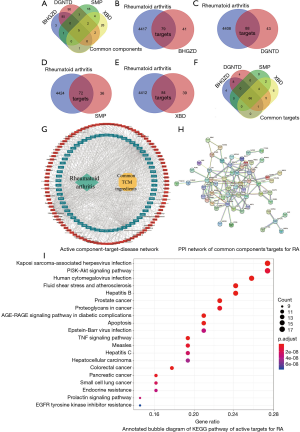
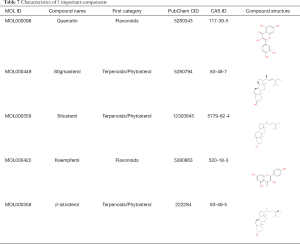
The TCMSP database was used to retrieve 30 TCM ingredients, and other platforms were used to retrieve 3 TCM ingredients. After screening out the repeated components, finally, BHGZD included 101 components, DGNTD included 197 components, SMP included 35 components, and XBD included 46 components. The 4 CHF had 5 common components, as shown in Figure 13A and Table 7.
Full table
Protein interaction network and key target information
After screening the targets obtained on the TCMSP and PharmMapper databases, we sorted out the related targets of the 4 CHF and let these targets intersect with RA related targets. The 4 CHF had 66 common targets for RA treatment, as shown in Figure 13B,C,D,E,F. A network of “active ingredients-targets-disease” is shown in Figure 13G. The protein interaction network and 58 key targets are shown in Figure 13H and Table S6. Node proteins were closely related to inflammatory factors, neovascularization, tumor cells, and so on.
KEGG enrichment analysis of key targets
We used R 4.0.2 software to perform KEGG enrichment analysis on the common targets of the 4 CHF for the treatment of RA. The KEGG results showed that the signaling pathways involved in RA treatment were mainly inflammation-related pathways, such as the TNF signaling pathway, and PI3k-Akt signaling pathway, and so on (Figure 13I).
Discussion
Description of research findings
By collating clinical guidelines and books related to RA or damp-heat type Bi syndrome, we conducted a pairwise meta-analysis and NMA to summarize the efficacy and safety of 4 highly recommended CHF combined with 1 csDMARD in treating RA. The evaluation and ranking were carried out according to the comparison results. In this study, after comprehensively assessing all the outcomes, SMPPD seemed to have a double guarantee of safety and efficacy. Besides, in the selection of Chinese patent medicines, an expert consensus (47) had also shown that more than 50% of experts recommended SMP (accounting for 53.6%) for the treatment of active RA, and another systematic review had reported that in the treatment of RA, SMP combined with western medicine could effectively reduce the inflammatory reaction, improve drug tolerance, and relieve joint symptoms (16).
In terms of TCM clinical effective rate, the statistical results of NMA showed that compared with the csDMARD alone, 4 CHF combined with 1 csDMARD could partly improve the ER, which was consistent with the results of direct comparisons. The changes of ESR and CRP were also important serological indicators to help diagnose the curative effect or evaluate the disease activity. In this study, the pairwise meta-analysis indicated that CHF + csDMARD could reduce inflammatory markers, but the NMA results demonstrated that only SMPPD and XBDPD could reduce the inflammatory levels of ESR and CRP. The differences between the 2 results also alerted us to the bias results caused by a small sample size or other factors. Meanwhile, due to the insufficient sample of other outcomes, we could not carry out NMA to evaluate and rank the other outcomes. Also, because the results of TSA suggested that our studies had too low samples, the results of meta-analysis might have had false-positive results among the 4 treatment groups. Therefore, whether CHF could reduce the levels of RF, IL-1, and TNF-α, and improve limb symptoms, such as TJC, SJC and MST, still needs to be further supported by the inclusion of more high-quality studies. Moreover, due to the differences in trial designs and small sample sizes, the results with high clinical heterogeneity should be treated cautiously. Based on the ER, ESR, and CRP measurement, the ranking results of this study indicated that XBDPD and SMPPD had potential advantages in anti-inflammatory and analgesic effects. In the results of cluster analysis, XBDPD, BHGZDPD, and SMPPD clearly stated a synergistic effect. This result was consistent with the many recommendations of the guidelines. Also, the NMA results showed that there was no statistical difference in the efficacy of the 4 CHF, which might be related to the presence of the same active ingredients among the 4 decoctions. For example, common ingredients included quercetin, stigmasterol, sitosterol, kaempferol, β-sitosterol, and so on, which have shown similar effects on regulating immunity and relieving joint symptoms in other studies (48).
However, the safety of drug administration was still an essential criterion for evaluating the treatment of RA. A lower incidence of ARs was associated with DGNTDPD and SMPPD, showing that they seemed to reduce toxic side effects or enhance the synergy of combination with 1 csDMARD. Moreover, the evidence of 9 retrieved systematic reviews (16,17,49-55) (Table S7) showed that ARs of each of the above CHF in treating rheumatic immune disease decreased. Nevertheless, both XBDPD and csDMARD had a high incidence of ARs, and the specific ARs were not recorded. Future studies need to report related ARs while demonstrating the efficacy. In addition, it is worth noting that Chinese medicines often cause ARs because of their abuse, but they are generally safe if used correctly (56). Therefore, we also need to pay great attention to the clinical application standard of TCM.
Common pharmacological actions
In this study, the 4 recommended CHF all reflected some evidence of efficacy. To explore the active ingredients and pharmacological effects of these classic prescriptions in the treatment of RA, we analyzed the 4 CHF under the perspective of systems biology and network pharmacology, especially exploring the common components, targets and signalling pathways. The results showed that the common ingredients in the 4 CHF were: quercetin, stigmasterol, sitosterol, kaempferol, and β-sitosterol. These 5 critical compounds might act on 66 vital targets such as ESR-1, RELA, EGFR, FOS, CCND-1, MAPK-8, NR3C-1, AR, IL-6, CASP-8, and so on, and are mainly enriched in the PI3k-Akt signaling pathway, TNF signaling pathway, prolactin signaling pathway, and so on. These targets and signalling pathways have been understood to play an important role in reducing inflammation, controlling hormones, regulating immunity, and slowing down joint destruction for a long time.
Previous experimental results have pointed out that sex hormones could affect the development of immune cells and have immunomodulatory effects (57). For example, estrogen receptor alpha (ESR1) has been related to the risk of inducing erosive arthritis (58), which was reflected in the fact that women were more likely to develop RA; and other studies (59) have shown that reduced androgen also meant an increased risk of RA. The synovial inflammatory response of RA was similar to the growth of tumors, showing aggressive hyperplasia and expressing epidermal growth factor receptor (EGFR) and its ligands (60). Studies (61)have also specified that EGFR plays a vital role in the process of osteoclast formation and synovial inflammation. Its activation (62) was shown to relate to the proliferation of RA synovial fibroblasts, and it participated in most of the process of RA development. Also, MAPK-8, as the target protein found in the Wnt signaling pathway, which is closely related to the pathogenesis of RA, was shown to be a vital signal transduction target that participates in various cell biological processes (63). At the same time, studies (64) have also shown that RELA and MAPK-8 might be mainly involved in immune cell transport and intercellular inflammatory signal transmission in RA treatment. Nowadays, cytokine-targeted therapy has gradually changed the results of many chronic inflammatory diseases. The cytokine IL-6 (65,66) affects the differentiation of T cells and B cells, as a critical driver of the acute phase response of RA, and has become a good target for RA treatment. Significantly, on the one hand, IL-6 might regulate joint inflammation and injury by influencing chondrocytes and osteoclasts; on the other hand, it might also mediate systemic inflammation caused by RA.
As a classical pathway for understanding and treating RA, the PI3K-Akt signaling pathway is considered to be involved in synovitis, cartilage destruction, bone erosion, and pannus (67,68). The TNF signaling pathway is also a classic pathway for the treatment of RA. For example, TNF-α inhibitors have been used clinically for many years and could antagonize the activity of pathogenic inflammatory factors or signal transduction pathway activators (69,70). Besides, in the research of the prolactin signaling pathway, we have observed that prolactin extensively acts on the proliferation and differentiation of various cells in the immune system, and is closely related to rheumatic immune diseases (71). Prolactin (72,73) could prevent chondrocyte apoptosis, increase trabecular bone area, prevent bone destruction and bone loss, and reduce the expression of proinflammatory cytokines in the synovium. However, the shared mechanism of these 4 prescriptions for clearing dampness and heat in RA treatment has not been thoroughly studied. Therefore, in future research, we could further study their common components, targets, and pathways through pharmacological and clinical experiments.
Limitations and prospects
This NMA had some limitations and prospects:
- The RCTs included were all Chinese studies, and the sample size was small, which could easily have led to publication bias, and exaggerated the publication of positive results (as shown by the TSA, Egger’s test, and Trim-Fill analysis results). Meanwhile, there were some defects in the experimental design, such as randomization method, allocation process, measurement standard and so on. Therefore, the results need to be further confirmed by a large sample and high-quality research.
- In the clinical setting, csDMARDs would also be used in combination. However, our study lacked relevant RCTs for comparison. The NMA should include some multi-arm studies for analysis and exploration in the future.
- Most studies did not report the internationally recognized criteria for disease activity, such as the ACR20/50/70 and DAS-28 scores. Other serological outcomes and radiological examinations should also be observed in addition to the subjective measurement of symptoms and signs.
- The lack of standardization between TCM prescriptions, such as differences in drug composition, dosage form, and dose, will cause the existence of clinical heterogeneity, and maybe lead to biased results of publication. Therefore, in future research, we should incorporate the distinction between granules, pills, and decoctions, and strictly follow the dosage standard of pharmacopoeia.
- The safety reports of CHF were insufficient, and the ARs were not described in detail. The relationship between the side effects and drug combination should be explored in the future. Moreover, the feasible and cost-effective safety detection methods of TCM should be further developed in the future. The public media should report the efficacy of TCM correctly and report the safety comprehensively to reduce misleading. Meanwhile, we should improve the rules and regulations of important quality control and use.
- The common pharmacological mechanisms of recommended CHF in the treatment of RA had not been confirmed, especially the compounds included in the database were not comprehensive, and the related targets were not updated in time. Therefore, the potential mechanism needs to be further confirmed through pharmacological and clinical trials.
Conclusions
Considering all the outcomes, this systematic review and NMA showed that SMP + csDMARD might be a more productive choice in relieving inflammation, reducing AEs, and improving efficacy for RA patients. Compared with the csDMARD alone, the combination of the recommended CHF and 1 csDMARD had a particularly better curative effect. However, due to the limitations of this study, further extensive, rigorous, standardized, and high-quality research is required for confirmation. For the study of the potential mechanisms, we found that in the treatment of RA, the 4 CHF had common components, targets, and pathways to regulate immune and inflammatory responses. Nevertheless, we still need more corresponding basic research to verify and explain these findings.
Acknowledgments
The authors acknowledge PubMed, Cochrane Library, EMBASE, CNKI, Wanfang, CQVIP, TCMSP, TCMID, PharmMapper, UniProt, Genecards, OMIM and STRING databases for providing their platforms and contributors for uploading their meaningful datasets. We also thank J. Jones and J. Chapnick for supporting this work as the English language editors.
Funding: This research was supported by National Natural Science Foundation of China Project (81573883); Military Logistics Research Project (CGZ16C007); Science and Technology Projects of Guangzhou (201704020160).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-445
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-445). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sparks JA. Rheumatoid Arthritis. Ann Intern Med 2019;170:ITC1-ITC16. [Crossref] [PubMed]
- Pisetsky DS. Advances in the Treatment of Rheumatoid Arthritis: Costs and Challenges. N C Med J 2017;78:337-40. [PubMed]
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094-108. [Crossref] [PubMed]
- Bullock J, Rizvi SAA, Saleh AM, et al. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med Princ Pract 2018;27:501-7. [Crossref] [PubMed]
- Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis 2019;78:1463-71. [Crossref] [PubMed]
- Wong PK. Medication adherence in patients with rheumatoid arthritis: why do patients not take what we prescribe? Rheumatol Int 2016;36:1535-42. [Crossref] [PubMed]
- Vallerand IA, Patten SB, Barnabe C. Depression and the risk of rheumatoid arthritis. Curr Opin Rheumatol 2019;31:279-84. [Crossref] [PubMed]
- Lü S, Wang Q, Li G, et al. The treatment of rheumatoid arthritis using Chinese medicinal plants: From pharmacology to potential molecular mechanisms. J Ethnopharmacol 2015;176:177-206. [Crossref] [PubMed]
- Daily JW, Zhang T, Cao S, et al. Efficacy and Safety of GuiZhi-ShaoYao-ZhiMu Decoction for Treating Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Altern Complement Med 2017;23:756-70. [Crossref] [PubMed]
- Xing Q, Fu L, Yu Z, et al. Efficacy and Safety of Integrated Traditional Chinese Medicine and Western Medicine on the Treatment of Rheumatoid Arthritis: A Meta-Analysis. Evid Based Complement Alternat Med 2020;2020:4348709 [Crossref] [PubMed]
- Jiang M, Zha Q, He Y, et al. Risk factors of gastrointestinal and hepatic adverse drug reactions in the treatment of rheumatoid arthritis with biomedical combination therapy and Chinese medicine. J Ethnopharmacol 2012;141:615-21. [Crossref] [PubMed]
- Fan WQ, Ma L, Wu J, et al. The Study on Correlation Between TCM Type and Disease Activity Index of Rheumatoid Arthritis. Hebei TCM 2017;37:1395-7.
- Hou L, Yuan XL, Zeng P, et al. Correlation Analysis Between TCM Syndrome Types and Disease Activity Indexes of Rheumatoid Arthritis. Arthritis Rheum 2018;7:5-8.
- Li Q. Study on correlation between TCM Syndrome Types and Laboratory indexes in patients with rheumatoid arthritis. Hunan University of TCM 2016.
- Wang PY, Chen MZ, Zhao RGT. Analysis of TCM syndromes and clinical indicators of 740 patients with rheumatoid arthritis. Hebei TCM 2019;41:342-6.
- Wang H, Huang Y, Shen P, et al. Modified Si-Miao Pill for Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med 2020;2020:7672152 [PubMed]
- Lam MY. Meta-anlysis on Clinical Efficacy of Classical Prescription in Treating Rheumatoid Arthritis. Nanjing Univ Tradit Chin Med 2018.
- Wang W, Zhou H, Liu L. The role of Chinese herbal medicine in the management of adverse drug reactions of leflunomide in treating rheumatoid arthritis. Phytomedicine 2020;68:153136 [Crossref] [PubMed]
- Zhou YY, Xia X, Peng WK, et al. The Effectiveness and Safety of Tripterygium wilfordii Hook. F Extracts in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front Pharmacol 2018;9:356. [Crossref] [PubMed]
- Huang Y, Wang H, Chen Z, et al. Efficacy and safety of total glucosides of paeony combined with methotrexate and leflunomide for active rheumatoid arthritis: a meta-analysis. Drug Des Devel Ther 2019;13:1969-84. [Crossref] [PubMed]
- Liu W, Qian X, Ji W, et al. Effects and safety of Sinomenine in treatment of rheumatoid arthritis contrast to methotrexate: a systematic review and Meta-analysis. J Tradit Chin Med 2016;36:564-77. [Crossref] [PubMed]
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. [Crossref] [PubMed]
- Higgins J, Green SE. Cochrane handbook for systematic reviews of interventions Version 5.1.0. . Cochrane Collab 2010:202-6.
- Tian JW, Li L. Network meta-analysis method and Practice. The Medicine Science and Technology Press of China: 2017.
- Jansen JP, Crawford B, Bergman G, et al. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health 2008;11:956-64. [Crossref] [PubMed]
- Han SY, Hong ZY, Xie YH, et al. Therapeutic effect of Chinese herbal medicines for post stroke recovery: A traditional and network meta-analysis. Medicine 2017;96:e8830 [Crossref] [PubMed]
- Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654 [Crossref] [PubMed]
- Asimit J, Day-Williams A, Zgaga L, et al. An evaluation of different meta-analysis approaches in the presence of allelic heterogeneity. Eur J Hum Genet 2012;20:709-12. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629-34. [Crossref] [PubMed]
- Jennions MD, Møller AP. Publication bias in ecology and evolution: an empirical assessment using the 'trim and fill' method. Biol Rev Camb Philos Soc 2002;77:211-22. [Crossref] [PubMed]
- Tunaru R. Hierarchical Bayesian models for multiple count data. Aust J Stat 2002;31:221-9.
- Zhang WF. Clinical observation of Simiaosan jiawei in the treatment of rheumatoid arthritis of dampness-heat blockage type. Fujian Univ Tradit Chin Med 2019.
- Yuan L, Wu JY, Tang J, et al. Clinical observation of Baihu plus Guizhi Decoction combined with Western medicine in treating rheumatoid arthritis with rheumatic heat arthralgia syndrome. J Liaoning Univ Tradit Chin Med 2019;21:168-71.
- Zhu Q, Zhao NN, Sui KY. Clinical observation on active rheumatoid arthritis treated with integrated traditional Chinese and Western Medicine. J Pract Tradit Chin Med 2014;30:25-6.
- Pang J, Xu KQ. Short term effect of modified Xuanbi decoction combined with methotrexate in the treatment of active rheumatoid arthritis. Chin Naturopathy 2010;18:58-9.
- Ma XY. Clinical Study of Modified Baihu and Guizhi Tang in the Treatment of Damp Heat Blockage Syndrome of Rheumatoid Arthritis. Acta Chin Med 2016;31:1573-7.
- Jiang H, Zhang YP. Study on the curative effect of Danggui Niantong decoction combined with methotrexate in the treatment of rheumatoid arthritis. Contemporary Medical Symposium 2020;18:200-1.
- Ding CH, Gao XM, Yang YS, et al. Clinical effect of Modified Danggui Niantong Decoction on rheumatoid arthritis of dampness heat obstruction type. Digest World Latest Med Inf 2017;17:163-4. (Electronic Version).
- Ge L, Shi ZM. Randomized controlled study of Danggui Niantong decoction combined with methotrexate in the treatment of rheumatoid arthritis. Acta Chin Med Pharm 2017;45:84-6.
- Zheng HZ, Da N, Huang M. Clinical observation on treating 30 cases of rheumatoid arthritis with Danggui Niantong Decoction. J Sichuan Tradit Chin Med 2015;33:102-3.
- Sheng H. Clinical Observation On Dang Gui Nian Tong Tang treatment of the hot and humid Blockage of rheumatoid arthritis (early). Anhui Univ Tradit Chin Med 2013.
- Li H. Treating 36 cases of rheumatoid arthritis of the Shire Bizu type with Simiao Wan plus western medicine. Clin J Chin Med 2017;9:31-3.
- Yang B, Liang QH, Wu D, et al. Clinical observation on Simiao Pills combined with Western medcine for 20 cases of rheumatoid arthritis in active stage. J Tradit Chin Med 2011;52:1566-9.
- Li AM, Gao P. Therapeutic effect of Modified Simiao powder on rheumatoid arthritis. Int J Trad Chin Med 2014;36:164-5.
- Wang JS, Ma W. Clinical observation on 80 cases of rheumatoid arthritis treated with modified Xuanbi Decoction and Western Medicine. Xinjiang J Tradit Chin Med Pharm 2008;26:16-8.
- Li M. Si Miao mixture for rheumatoid arthritis dampness-heat stagnation syndrome clinical curative effect and research on the effects of serum IL-1, TNF-α levels. Hunan Univ Tradit Chin Med 2016.
- Zhao J, Zha Q, Jiang M, et al. Expert consensus on the treatment of rheumatoid arthritis with Chinese patent medicines. J Altern Complement Med 2013;19:111-8.
- Qian H, Jin Q, Liu Y, et al. Study on the Multitarget Mechanism of Sanmiao Pill on Gouty Arthritis Based on Network Pharmacology. Evid Based Complement Alternat Med 2020;2020:9873739 [Crossref] [PubMed]
- Du MR, Guo ZZ, Fen FH. Meta analysis of clinical efficacy and safety of Simiao powder in the treatment of acute gouty arthritis. Tradit Chin Med Res 2015;25:66-70.
- Du MR, Guo ZZ, Fen FH. Systematic Evaluation on Efficacy and Safety of Simiao San as Main Prescription for Gouty Arthritis. Chin J Exp Tradit Med Form 2015;21:212-6.
- Hu YR, Liu XS, Liu JG, et al. Meta-analysis of Danggui Niantong Decoction in the treatment of rheumatoid arthritis. Gansu Med J 2021;40:133-8.
- Li P, Wang YJ, Cao Y. Meta-analysis of the Efficacy and Safety of Simiao Decoction on Gout Compared with Colchicine. J Emerg Tradit Chin Med 2019;28:449-52.
- Ping F, Li CY, Zhu FL, et al. Meta-analysis of Curative Effect of Xuanbi Tang in Treating Gouty Arthritis. Chin J Exp Tradit Med Form 2015;21:193-6.
- Yi J. Meta analysis of Modified Simiao powder combined with external application of traditional Chinese medicine in the treatment of gouty arthritis. Liaoning Univ tradit Chin Med 2017;
- Zhou QY, Lu L, Cui JK, et al. A Systematic Review and Meta-Analysis of Simiao Powder in Treatment of Gout Arthritis and Uric Acid. Liaoning J tradit Chin Med 2016;43:1356-60.
- Zheng WR, Li EC, Peng S, et al. Tu Youyou winning the Nobel Prize: Ethical research on the value and safety of traditional Chinese medicine. Bioethics 2020;34:166-71. [Crossref] [PubMed]
- Martocchia A, Stefanelli M, Cola S, et al. Sex steroids in autoimmune diseases. Curr Top Med Chem 2011;11:1668-83. [Crossref] [PubMed]
- Sánchez-Maldonado JM, Cáliz R, Canet L, et al. Steroid hormone-related polymorphisms associate with the development of bone erosions in rheumatoid arthritis and help to predict disease progression: Results from the REPAIR consortium. Sci Rep 2019;9:14812. [Crossref] [PubMed]
- Gubbels Bupp MR, Jorgensen TN. Androgen-Induced Immunosuppression. Front Immunol 2018;9:794. [Crossref] [PubMed]
- Yuan FL, Li X, Lu WG, et al. Epidermal growth factor receptor (EGFR) as a therapeutic target in rheumatoid arthritis. Clin Rheumatol 2013;32:289-92. [Crossref] [PubMed]
- Liu D, Yan J, Guo M, et al. Effect of methotrexate combined with Sanhuang Yilong decoction on serum and synovial fluid aquaporin levels in rheumatoid arthritis dampness-heat blockage syndrome. J Tradit Chin Med 2018;38:618-24. [Crossref] [PubMed]
- Li Z, Xu M, Li R, et al. Identification of biomarkers associated with synovitis in rheumatoid arthritis by bioinformatics analyses. Biosci Rep 2020;40:BSR20201713 [Crossref] [PubMed]
- Rabelo FS, da Mota LM, Lima RA, et al. The Wnt signaling pathway and rheumatoid arthritis. Autoimmun Rev 2010;9:207-10. [Crossref] [PubMed]
- Song X, Zhang Y, Dai E, et al. Prediction of triptolide targets in rheumatoid arthritis using network pharmacology and molecular docking. Int Immunopharmacol 2020;80:106179 [Crossref] [PubMed]
- Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin Immunopathol 2017;39:365-83. [Crossref] [PubMed]
- Choy EH, De Benedetti F, Takeuchi T, et al. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol 2020;16:335-45. [Crossref] [PubMed]
- Yu Z, Xu H, Wang H, et al. Foxc1 promotes the proliferation of fibroblast-like synoviocytes in rheumatoid arthritis via PI3K/AKT signalling pathway. Tissue Cell 2018;53:15-22. [Crossref] [PubMed]
- Zhang H, Bao GF, Cui ZM. Research progress of PI3k-Akt signaling pathway in the pathogenesis of rheumatoid arthritis. J Southeast Univ 2019;38:358-62.
- Donahue KE, Gartlehner G, Schulman ER, et al. AHRQ Comparative Effectiveness Reviews. Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018.
- Cai P, Jiang T, Li B, et al. Comparison of rheumatoid arthritis (RA) and osteoarthritis (OA) based on microarray profiles of human joint fibroblast-like synoviocytes. Cell Biochem Funct 2019;37:31-41. [Crossref] [PubMed]
- Borba VV, Zandman-Goddard G, Shoenfeld Y. Prolactin and Autoimmunity. Front Immunol 2018;9:73. [Crossref] [PubMed]
- Ledesma-Colunga MG, Adán N, Ortiz G, et al. Prolactin blocks the expression of receptor activator of nuclear factor κB ligand and reduces osteoclastogenesis and bone loss in murine inflammatory arthritis. Arthritis Res Ther 2017;19:93. [Crossref] [PubMed]
- Adán N, Guzmán-Morales J, Ledesma-Colunga MG, et al. Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis. J Clin Invest 2013;123:3902-13. [Crossref] [PubMed]




