
Interhemispheric resting-state functional connectivity abnormalities in type 2 diabetes patients
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic progressive metabolic disease characterized by hyperglycemia. Diabetes can result in progressive damage to the central nervous system. Approximately 10.8–17.5% of diabetic patients develop cognitive dysfunction (1), including mild cognitive impairment (MCI) and dementia (2). T2DM is associated with cognitive impairment (3,4), which typically symptoms manifest as a decline in memory, attention, information processing speed (5), and executive function (6). T2DM-induced neurocognitive changes may significantly affect quality of life. However, its exact neuropathophysiological substrate has not yet been fully elucidated.
The advent of novel neuroimaging techniques has shed new light on this issue. Conventional MRI is used to show changes in human brain structure to reflect the corresponding pathological changes. Resting-state functional magnetic resonance imaging (rs-fMRI) is a non-invasive procedure based on blood-oxygen-level-dependent (BOLD) neuroimaging technology, providing a way to directly characterize the functional connectivity between brain regions and quantify interhemispheric functional interactions and being useful for assessing neural-network connectivity (7). Rs-fMRI studies have shown specific brain network changes in T2DM patients, especially in the default mode network (DMN), which may suggest a heightened risk for cognitive decline (8,9). The posterior cingulate cortex (PCC), which is associated with insulin resistance in selected brain regions, has developed abnormal functional connectivity in T2DM patients (10). Chen et al. (11) found that T2DM patients showed reduced functional connectivity between the bilateral hippocampus. These studies suggest that the decline in cognitive performance in T2DM patients is associated with reduced functional connectivity. However, previous studies using rs-fMRI have primarily focused on the abnormal functional connectivity between brain regions. The functional coordination between bilateral hemispheres in T2DM has not yet been directly examined.
Functional homotopy, the high degree of synchronization of spontaneous activity between geometrically corresponding hemispheric (i.e., homotopy) regions, is a fundamental feature of the brain’s internal functional structure (12). Many early studies have also shown that the mode of information communication between bilateral hemispheres is a key component of cognitive processing (13,14). Based on the importance of information communication and integration between the two hemispheres for higher-order cognitive function, and multiple cases of cognitive impairment in T2DM patients (3,4), we hypothesized that the information communication mode between hemispheres in T2DM patients is abnormal.
To evaluate the spatial heterogeneity of functional information exchange and integration between the rest cerebral hemispheres between T2DM patients and normal controls (NC), we analyzed the rs-fMRI data using voxel-mirrored homotopic connectivity (VMHC). VMHC is a method that directly compares interhemispheric resting-state functional connectivity (RSFC) (12). The VMHC can quantitatively evaluate the functional connection of time series correlation between the corresponding voxels mirrored on both sides of the brain. The VMHC has been used successfully to study functional homotopy in major depressive disorder (15), autism (16), and normal aging (12).
The aim of this study is to evaluate the abnormal homotopy functional connection in T2DM patients, so as to provide evidence for whether the change of functional information exchange and integration between hemispheres of T2DM patients is involved in the pathogenesis of T2DM-related cognitive dysfunction. In addition, considering the importance of information exchange and integration between cerebral hemispheres for cognitive function, it is necessary to explore whether there is a correlation between VMHC and neuropsychological tests in T2DM patients.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1655).
Methods
Participants
This study protocol was approved by an independent Ethics Committee of the 980th Hospital of Joint Logistics Support Force (No.: 2020-KY-38), China. Before this study, all the subjects voluntarily signed the written informed consent. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The complete medical records of each subject were also obtained. The privacy rights of human subjects were always observed. All participants were right-handed. Four T2DM patients and two NC subjects were excluded from subsequent analyses as a result of head motion artifacts.
A total of 69 T2DM patients from the Departments of Endocrinology were enrolled in this study, based on the American Diabetes Association (ADA) diagnosis criteria (17). All T2DM patients were routinely treated with hypoglycemic drugs. Additionally, the T2DM patients were treated with other non-pharmacologic strategies such as diet control and exercise. All procedures were completed under the supervision of an experienced endocrine doctor.
Also enrolled were a total of 69 NC subjects matching for age, sex and education level with the T2DM group. All 69 NC subjects were agreed to perform MRI. The NC subjects with fasting glucose >6.1 mmol/L or postprandial glucose >7.8 mmol/L were excluded.
The exclusion criteria for all participants were central nervous system diseases, including a history of cerebral infarction, history of head trauma, history of drug dependence, history of alcohol dependence, and depression (assessed by Hamilton Depression scale); other neurological or psychiatric illnesses (excluded through clinical evaluation and medical history); contraindications for magnetic resonance imaging (MRI); and dementia [assessed by Mini-Mental State Examination (MMSE) score <24]. Patients with related medical diseases (such as cancer, diabetic ketoacidosis and hypothyroidism) and severe vision or hearing loss were also excluded from this study. Patients with vascular risk factors [hyperlipidemia, white matter hyperintensity (WMH), hypertension] were also excluded.
Neuropsychological tests
In this study, the MMSE and the Montreal Cognitive Assessment (MoCA) scale were used to test the overall cognitive function of the subjects. The MMSE scale is the most widely used scale for testing possible dementia (18). The MoCA scale (Beijing version) was used to evaluate the cognitive status (including visual space, executive function, attention, language, delayed recall and orientation function) of each subject (19). A higher MOCA score (from 0 to 30) indicates better cognitive function. Approximately 1 hour was needed to complete all the tests in an established order. A skilled neuropsychiatrist assisted the test process with a single blind method.
MRI data acquisition
All the MRI data were acquired with a 3.0 T Siemens Tim Trio MRI scanner (Erlangen, Germany), which is equipped with a standard eight-channel head coil. First, T1-weighted, T2-weighted and fluid attenuation-inversion recovery imaging data was collected from all subjects to exclude organic disease and WMH lesions. The rs-fMRI data were then acquired using an echo-planar imaging (EPI) sequence. The rs-fMRI detailed parameters are as follows: 36 axial slices, slice thickness =3 mm, repetition time (TR) =2,000 ms, echo time (TE) =30 ms, flip angle (FA) =90°, field of view (FOV) =192×192 mm2, data matrix =64×64, voxel size =3×3×3 mm3, and total volumes =240. Rs-fMRI scanning of each participant lasted for 8 minutes. During the rs-fMRI scan, all subjects kept their eyes closed and their bodies still, tried not to think, and avoided falling asleep. A high-resolution sagittal structural T1-weighted anatomical sequence was then acquired using the following parameters: TR =1,900 ms, TE =2.52 ms, FA =9°, slice thickness =1 mm, FOV =256 mm × 256 mm, matrix =256×256, voxel size =1×1×1 mm3, and 176 slices.
Functional image preprocessing
The rs-fMRI data were preprocessed using the Data Processing Assistant for rs-fMRI (DPARSF) (20) with statistical parametric mapping (SPM8, http://wwwfil.ion.ucl.ac.uk/spm) in MATLAB (R2010a) (MathWork, Natick, MA, USA). Firstly, after the functional and structural image data of DICOM format were converted into NifTI format, the data of the first 10 time points were deleted to ensure that the subjects were not affected by ambient noise during MRI scanning. Secondly, slice timing was performed. Third, realignment for head motion correction was performed. Any subjects with head motion >2.0 mm translation or >2.0° rotation in any direction were excluded (20). Linear regression was used to remove covariant parameters such as average signals of whole brain, cerebrospinal fluid, white matter, the signal from a ventricular region of interest (ROI) and head movement parameters (21). The functional images were registered to the standard MNI space, and resampled with the pixel size of 3×3×3 mm3. The generated images were smoothed with a Gaussian kernel having a full-width at half-maximum (FWHM) value of 6 mm (FWHM equals two times the voxel size) (12). Linear trend removal and bandpass (0.01–0.08 Hz) filtering were conducted using the Resting-State fMRI Data Analysis Toolkit (REST) (22). After filtering, the generated image data were used for VMHC analysis.
VMHC
VMHC whole brain analysis of the data between the two groups was carried out using DPARSF software. For each subject, the VMHC value was determined by calculating the Pearson correlation coefficient between each voxel in one hemisphere and the mirrored time series in the contralateral hemisphere. The correlation values were then transformed by Fisher’s z to improve their normal distribution. VMHC value can be used for group analysis statistics. A previous study describes the details of VMHC acquisition (12).
The mean framewise displacement (FD) that indicated the temporal derivative of the motion parameters were computed, as slight head motion could affect RSFC results (23). Differences in mean FD values were subsequently compared between T2DM patients and NC subjects using independent-sample t-tests. Thereafter, the computed FD values were used as the covariates for the VMHC comparisons among groups.
Statistical analysis
The PASW statistical software package (version 18.0, Chicago, IL, USA) was used to conduct all analyses. The normal distribution of continuous variables was tested using PASW. The differences of demographic data, clinical measurement index, and neuropsychological scale between T2DM patients and the NC subjects were analyzed by independent sample t-test. The sex differences between the two groups were compared using Chi-square test.
Here we compared the VMHC maps by two-sample t-tests after controlling for age, sex and education level. Monte Carlo simulation function was utilized to determine the exact level of statistical significance. After the AlphaSim correction, the threshold for single voxel was down to 0.01 with a 40-voxel cluster. All operations were performed using REST.
The 7 identified clusters (see Table 1), which resulted from regions showing significant differences in VMHC between T2DM patients and NC subjects, were defined as the ROIs. To determine whether VMHC calculations were relevant to cognitive decline, the correlation between the MOCA values and the VMHC values of each ROI was tested using partial correlation analysis (after controlling for age, sex and education level) with statistical significance set at 0.05. A total of 7 ROIs were enrolled into the test. Bonferroni correction was used to determine the statistical significance level when multiple tests were performed. Therefore, the statistical significance level of each single test was set at 0.05/7 (total significance level/the number of multiple tests).

Full table
Results
Demographic information and clinical characteristics
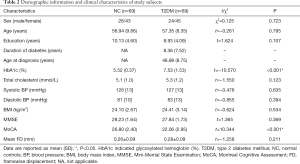
Table 2 shows the demographic, clinical and neuropsychological test characteristics of all the subjects. No significant differences (all P>0.05) were found in age, sex or education level between the T2DM patients and NC groups. The T2DM patients and NC groups did not differ (all P>0.05) in terms of total cholesterol level, blood pressure, MMSE score and body mass index (BMI). Compared with the NC subjects, T2DM patients had higher HbA1c level (P<0.001). T2DM patients had significantly decreased MoCA scores than NC subjects (P<0.001). No significant difference (P=0.211) was found in the mean FD values between T2DM patients and NC subjects.
Full table
VMHC differences between groups
When compared with the NC group, patients with T2DM demonstrated significant decreases in VMHC (Figure 1) in the medial orbitofrontal gyrus cortex (mOFC), anterior cingulate gyrus, inferior parietal lobe, superior temporal gyrus and middle temporal gyrus (MTG), which are part of the DMN regions (24). Several non-DMN regions also exhibited decreased VMHC, including the middle occipital gyrus and superior occipital gyrus. Moreover, the cerebellum posterior lobe and tonsil and the inferior temporal gyrus exhibited increased VMHC (Figure 2). More details on these regions are provided in Table 1.
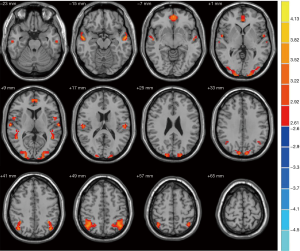
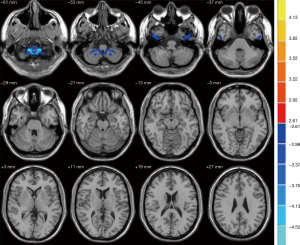
Relationships between VMHC and MoCA
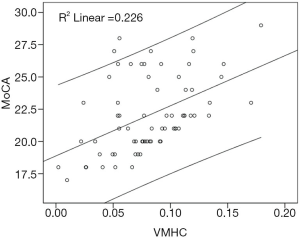
Partial correlation analysis indicated a significant positive correlation (r=0.335, P=0.006) between the MoCA and VMHC of the MTG in T2DM patients (Figure 3). There was no significant correlation between other brain regions and MoCA scores in T2DM patients. Partial correlation analysis also indicated no significant difference (P=0.286) between the VMHC and MoCA scores of the MTG in NC subjects.
Discussion
This study compared interhemispheric RSFC changes between T2DM patients and NC subjects via the VMHC method, and demonstrated that T2DM patients had decreased VMHC in DMN regions. We found that the MTG is related to poor cognitive performance, which could be the critical node to understanding cognitive impairment in T2DM. In contrast, T2DM patients exhibited increased VMHC in the cerebellum and inferior temporal gyrus.
In this study, there was no significant difference observed in the MMSE scores between T2DM patients and NC subjects. However, the MoCA scores of T2DM patients were significantly lower than those of NC subjects. Because T2DM patients were relatively younger and their disease durations were not long enough, the degree of cognitive decline did not seem to reach the level of dementia or Alzheimer’s disease (AD). The MoCA was developed as a relatively rapid, brief screening instrument for MCI and AD. The MoCA was considered a more sensitive measure than the MMSE (19,25). Thus, the T2DM patients we recruited may be mainly patients with MCI.
The disturbance of the interhemispheric RSFC within DMN regions provides various dimensions of neuroimaging support for the findings of previous studies into role of T2DM in cognitive neurodegeneration. The resting-state DMN had consistently higher blood oxygen level dependent (BOLD) activity than other cognitive activities in several brain regions, including the MTG, middle frontal gyrus, PCC, anterior cingulate cortex, and inferior parietal lobe (24). Interestingly, previous studies have also demonstrated reduced functional connectivity within the DMN in T2DM patients (9,26), suggesting considerable similarity to results reported in AD populations (27,28). Although the mechanism of high incidence of AD in T2DM is not clear, the following reasons can be used to explain the results of this study. The default network brain regions are areas with high metabolic activity and increased aerobic glycolysis, which makes it easier for amyloid to deposit in these brain regions (29). The inherent disorder of glucose metabolism in diabetes may accelerate this process (26).
Remarkably, the disturbance in interhemispheric RSFC in the MTG showed by fMRI could play a key role in evaluating cognitive dysfunction in T2DM. Although previous studies have confirmed structural or functional impairment in various brain regions of T2DM patients, the most consistently reported altered brain region in the T2DM population is the MTG (30,31). Furthermore, Musen et al. and Chen et al. found that the PCC exhibited reduced functional connectivity with the MTG in T2DM compared with NC subjects (10,26). Xia et al. (32) found that T2DM patients had significantly decreased amplitude of low frequency (ALFF) values in MTG, which may play a central role in cognitive decline associated with T2DM. Thus, to further understand the brain mechanisms of cognitive impairment, we performed a correlation analysis relating MTG impairment and cognitive decline. After applying Bonferroni correction, a significant positive correlation (r=0.335, P=0.006) was observed between the VMHC and MoCA scores of the MTG in T2DM patients, but no significant difference (P=0.286) was observed in NC subjects. Our results add new insights to the results reported by Xia et al. (32).
Compared with NC subjects, T2DM patients also exhibited reduced VMHC in other non-DMN brain regions, such as the middle occipital gyrus and superior occipital gyrus. Cui et al. also found significantly decreased ALFF and regional homogeneity (ReHo) values in the occipital lobe of T2DM patients (33). Previous fMRI studies have shown that the decrease of neural activity in the occipital lobe is related to the impairment of cortical visual processing (34). It is now widely accepted that diabetes is associated with retinopathy, which can lead to visual impairment. In this study, there were no clinical visual changes in T2DM patients, which may indicate that the decreased VMHC in the occipital lobe may be an early change before the appearance of clinically measurable visual symptoms.
Notably, in the present study, the cerebellum posterior lobe and tonsil and inferior temporal gyrus exhibited increased VMHC in T2DM patients. Previous anatomical and functional neuroimaging studies and clinical evidence obtained from the treatment of patients with cerebellar lesions have suggested that the cerebellum plays a role in cognitive (i.e., attention, speech learning, memory and cognitive planning) and affective processes, in addition to fine motor and muscle tension coordination (35-37). Furthermore, we found a significant decrease in VMHC in the mOFC of T2DM patients, which has not been reported in any previous functional imaging study of T2DM. The mOFC is part of the medial prefrontal cortex, which is related to social cognition function, especially emotion regulation (38). The inferior temporal gyrus plays an important role in speech fluency and cognitive function affected in the early stage of AD (39,40). A recent study indicated that the cerebellum has an anatomic relationship with the prefrontal cortex and limbic system (41). These regions form multiple closed-loop neural circuits that participate in cognitive and emotional processing (42). Thus, the increased VMHC observed in the cerebellum and inferior temporal gyrus might reflect a coordination mechanism needed to mobilize additional neurons to initiate or coordinate emotional and higher-order cognition. However, the underlying biological mechanism for the morphological differences observed in the cerebellum and inferior temporal gyrus requires further study.
The above results show that the information integration function of multiple mirror brain regions is impaired in T2DM patients. It has been reported in previous literature that metformin, rosiglitazone and insulin have been shown to be beneficial to the memory and cognition of T2DM patients (43-45). In addition to drug therapy, the protective effect of lifestyle intervention (diet, exercise) on cognitive function also showed a good effect (46). Therefore, we speculate that these interventions may also promote the recovery of information integration function in brain regions, given the importance of bilateral hemispheric coordination for cognitive functions.
This study had several limitations. First, the bilateral brain parenchyma is asymmetrical. In this study, symmetrical templates and smoothed functional image data are used to solve this problem. Future studies should avoid the possible bias. Secondly, this study design was cross-sectional. In the future, T2DM patients with dementia should be included for longitudinal neuroimaging studies to explore the longitudinal dynamic changes of VMHC in related brain regions. Finally, the results of this study were obtained when the participants were in a resting state. Future studies should combine resting-state and task-state fMRI simultaneously to investigate whether abnormal brain regions with VMHC in resting state are also damaged under task conditions.
In conclusion, T2DM patients develop aberrant interhemispheric RSFC. Decreased VMHC in the DMN provides new evidence of a functional substrate for the involvement of DMN regions in the pathogenesis of the neurocognitive changes associated with T2DM. The MTG is related to a decline in cognitive function in T2DM, suggesting that MTG impairment could serve as a key node within the DMN to recognize T2DM-related cognitive dysfunction. In contrast, increased VMHC was found in the cerebellum and inferior temporal gyrus, which might indicate a coordination effect in T2DM patients.
Acknowledgments
Funding: This work was supported by the Hebei Province Medical Science Research Key Project of China (No. 20191201).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist Available at https://dx.doi.org/10.21037/apm-21-1655
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1655
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1655). All authors report funding from the Hebei Province Medical Science Research Key Project of China (No. 20191201). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethics Committee of the 980th Hospital of Joint Logistics Support Force (No.: 2020-KY-38) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bruce DG, Casey GP, Grange V, et al. Cognitive impairment, physical disability and depressive symptoms in older diabetic patients: the Fremantle Cognition in Diabetes Study. Diabetes Res Clin Pract 2003;61:59-67. [Crossref] [PubMed]
- Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol 2008;7:184-90. [Crossref] [PubMed]
- Jacobson AM, Musen G, Ryan CM, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842-52. [Crossref] [PubMed]
- Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia 2005;48:2460-9. [Crossref] [PubMed]
- van den Berg E, Reijmer YD, de Bresser J, et al. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia 2010;53:58-65. [Crossref] [PubMed]
- Mogi M, Horiuchi M. Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ J 2011;75:1042-8. [Crossref] [PubMed]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8:700-11. [Crossref] [PubMed]
- Ishibashi K, Sakurai K, Shimoji K, et al. Altered functional connectivity of the default mode network by glucose loading in young, healthy participants. BMC Neurosci 2018;19:33. [Crossref] [PubMed]
- Zhou H, Lu W, Shi Y, et al. Impairments in cognition and resting-state connectivity of the hippocampus in elderly subjects with type 2 diabetes. Neurosci Lett 2010;473:5-10. [Crossref] [PubMed]
- Chen YC, Jiao Y, Cui Y, et al. Aberrant brain functional connectivity related to insulin resistance in type 2 diabetes: a resting-state fMRI study. Diabetes Care 2014;37:1689-96. [Crossref] [PubMed]
- Chen Z, Liu M, Liu M, et al. Nan Fang Yi Ke Da Xue Xue Bao 2014;34:1083-91. [Resting-state brain functional magnetic resonance imaging in patients with type 2 diabetes mellitus]. [PubMed]
- Zuo XN, Kelly C, Di Martino A, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 2010;30:15034-43. [Crossref] [PubMed]
- Compton RJ, Feigenson K, Widick P. Take it to the bridge: an interhemispheric processing advantage for emotional faces. Brain Res Cogn Brain Res 2005;24:66-72. [Crossref] [PubMed]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex 2008;18:2553-9. [Crossref] [PubMed]
- Wang L, Li K, Zhang QE, et al. Interhemispheric functional connectivity and its relationships with clinical characteristics in major depressive disorder: a resting state fMRI study. PLoS One 2013;8:e60191 [Crossref] [PubMed]
- Li Q, Becker B, Jiang X, et al. Decreased interhemispheric functional connectivity rather than corpus callosum volume as a potential biomarker for autism spectrum disorder. Cortex 2019;119:258-66. [Crossref] [PubMed]
- . American Diabetes A. Standards of medical care in diabetes--2010. Diabetes Care 2010;33:S11-61. [Crossref] [PubMed]
- Galea M, Woodward M. Mini-Mental State Examination (MMSE). Aust J Physiother 2005;51:198. [Crossref] [PubMed]
- Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry 2012;12:156. [Crossref] [PubMed]
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci 2010;4:13. [PubMed]
- Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005;102:9673-8. [Crossref] [PubMed]
- Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 2011;6:e25031 [Crossref] [PubMed]
- Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142-54. [Crossref] [PubMed]
- Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98:676-82. [Crossref] [PubMed]
- Whitney KA, Mossbarger B, Herman SM, et al. Is the montreal cognitive assessment superior to the mini-mental state examination in detecting subtle cognitive impairment among middle-aged outpatient U.S. Military veterans? Arch Clin Neuropsychol 2012;27:742-8. [Crossref] [PubMed]
- Musen G, Jacobson AM, Bolo NR, et al. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 2012;61:2375-9. [Crossref] [PubMed]
- Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A 2004;101:284-9. [Crossref] [PubMed]
- Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry 2010;67:584-7. [Crossref] [PubMed]
- Vlassenko AG, Vaishnavi SN, Couture L, et al. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci U S A 2010;107:17763-7. [Crossref] [PubMed]
- Brundel M, van den Heuvel M, de Bresser J, et al. Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci 2010;299:126-30. [Crossref] [PubMed]
- Zhang Y, Zhang X, Zhang J, et al. Gray matter volume abnormalities in type 2 diabetes mellitus with and without mild cognitive impairment. Neurosci Lett 2014;562:1-6. [Crossref] [PubMed]
- Xia W, Wang S, Sun Z, et al. Altered baseline brain activity in type 2 diabetes: a resting-state fMRI study. Psychoneuroendocrinology 2013;38:2493-501. [Crossref] [PubMed]
- Cui Y, Jiao Y, Chen YC, et al. Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes 2014;63:749-60. [Crossref] [PubMed]
- Liu Y, Liang P, Duan Y, et al. Abnormal baseline brain activity in patients with neuromyelitis optica: a resting-state fMRI study. Eur J Radiol 2011;80:407-11. [Crossref] [PubMed]
- Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci 1997;17:438-58. [Crossref] [PubMed]
- Allen G, Buxton RB, Wong EC, et al. Attentional activation of the cerebellum independent of motor involvement. Science 1997;275:1940-3. [Crossref] [PubMed]
- De Smet HJ, Paquier P, Verhoeven J, et al. The cerebellum: its role in language and related cognitive and affective functions. Brain Lang 2013;127:334-42. [Crossref] [PubMed]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 2006;7:268-77. [Crossref] [PubMed]
- Liu J, Zhang B, Wilson G, et al. New Perspective for Non-invasive Brain Stimulation Site Selection in Mild Cognitive Impairment: Based on Meta- and Functional Connectivity Analyses. Front Aging Neurosci 2019;11:228. [Crossref] [PubMed]
- Scheff SW, Price DA, Schmitt FA, et al. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 2011;24:547-57. [Crossref] [PubMed]
- Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev 2010;20:236-60. [Crossref] [PubMed]
- Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain 2006;129:290-2. [Crossref] [PubMed]
- Ryan CM, Freed MI, Rood JA, et al. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care 2006;29:345-51. [Crossref] [PubMed]
- Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 2005;13:950-8. [Crossref] [PubMed]
- Nathan DM, Berkwits M. Trials that matter: rosiglitazone, ramipril, and the prevention of type 2 diabetes. Ann Intern Med 2007;146:461-3. [Crossref] [PubMed]
- Yamamoto N, Yamanaka G, Takasugi E, et al. Lifestyle intervention reversed cognitive function in aged people with diabetes mellitus: two-year follow up. Diabetes Res Clin Pract 2009;85:343-6. [Crossref] [PubMed]
(English Language Editor: G. Stone)


