
Risk factors for oral mucositis in patients with malignant tumors: a prospective cohort study
Introduction
Malignant tumors are newly generated abnormal tissues formed by the abnormal proliferation of clones caused by loss of the normal regulation of cell growth at the gene level under the action of various carcinogenic factors. The worldwide incidence of cancer is on the rise and according to statistics, every year more than 10 million people suffer from malignant tumors, and more than 9 million die, making this the second leading cause of death (1). Surgery, chemotherapy, and radiotherapy are the main treatment methods for patients with malignant tumors, and can improve local control, reduce metastatic spread, and improve survival rates (2). However, while both chemotherapy and radiotherapy kill cancer cells, they also affect normal cells, which may lead to adverse reactions and complications. Oral mucositis is a common complication of radiotherapy and chemotherapy in patients with malignant tumors (3), and in severe cases, treatment must be interrupted or reduced, which has a negative impact on the therapeutic effect and prognosis (4,5). Oral mucositis is an inflammatory or ulcerative lesion of oral mucosa (6) which often manifests as local pain affecting chewing and eating. This may lead to malnutrition and decreased quality of life (7,8), as well as the risk of infection (9).
Shouval et al. found that age, lower body mass index, smoking, and the use of antibiotics and methotrexate were associated with the development of moderate to severe oral mucositis in allogeneic hematopoietic stem cell transplantation recipients (10). Çakmak et al.’s study (11) on cancer patients undergoing outpatient chemotherapy found that the incidence of oral mucositis was 51.7%, and advanced age, loss of appetite and long duration of chemotherapy were its risk factors. Dodd et al. (12) estimated the incidence of oral mucositis in chemotherapy patients to be 25.1% but found its causes and risk factors were not clear. Therefore, this study investigated the incidence of oral mucositis in patients with malignant tumors and explored the impact of factors, including age, gender, educational level, tumor diseases, tumor staging, radiotherapy, radiotherapy duration, chemotherapy, denture, smoking history, white blood cell count, oral cavity cleanness, body mass index, hemoglobin, creatinine, urea nitrogen, blood glucose, vomiting, diarrhea, and other factors on its risk. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1675).
Methods
Participants
Patients with malignant tumors who were hospitalized in the Department of Oncology Radiotherapy of the Affiliated Hospital of Nantong University from January 2020 to December 2020 were selected as the research subjects.
Inclusion criteria: (I) the diagnosis of malignant tumor was made according to the National Comprehensive Cancer Network (NCCN) guidelines; (II) the patient was over 18 years old; (III) the patient has no disturbance of consciousness and could communicate normally; (IV) the patient was fully informed and signed informed consent for their participation.
Exclusion criteria: patients with blood disease.
Sample size calculation
The calculated sample size was 60 cases, and the data of 74 patients were collected.
Assessment of oral mucositis and pH value
Patients who met the inclusion criteria signed the informed consent after admission, and data on their general condition was collected. The oral pH value of patients was then measured to evaluate the occurrence and grading of oral mucositis.
This saw the use of Geshan Precision Test Paper (Shanghai Linlei electronics co., LTD, China) in the morning before gargling and drinking water, before lunch, after siesta, and at night before bedtime (four times per day). A precision test paper was pasted on the median of the patient’s tongue surface and under the tongue, and after the paper was soaked, its color was observed, and its average value was taken to determine the pH value. Abnormal results were considered as those exceeding the normal value range (6.5–7.0).
Ethical considerations
The study was approved by the ethics board at the Affiliated Hospital of Nantong University and informed consent was taken from all the patients. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Research scales
Questionnaire on general information of inpatients
General information of inpatients was obtained through direct inquiry and through consulting medical records, and included gender, age, educational level, tumor type, clinical stage, denture, oral pH value, presence of oral diseases, smoking history, oral cleanliness, and chemotherapy drugs.
Oral mucositis grading scale
The oral mucositis grading scale (13) is based on the grading standard developed by the American Society of Radiotherapy Oncology (RTOG), and was divided into 0-IV grades according to the severity of oral mucositis as follows: Grade 0: no oral mucositis; Grade I: erythema of oral mucosa accompanied by mild pain; Grade II: oral mucosa erythema, ulceration, but the patient could eat; Grade III: oral mucosal ulcer, the patient could eat semi-liquid food; Grade IV: the ulcer was severe and the patient could not eat.
Oral cleanliness grading scale
The oral cleanliness score (14) was evaluated from eight aspects: mucous membrane, gums, tongue, odor, teeth, lips, injury, and palate. For grading the mucous membrane, 1point was awarded for wet and intact mucosa, 2 points for dry and intact mucosa, and 3 points for dry, abrased, or ulcerated mucosa. The condition of the gums was scored as 1 point for no bleeding and atrophy of the gums, 2 points for mild atrophy and hemorrhage, and 3 points when gums were atrophied, easily bled, and swollen. A wet tongue with a small amount of tongue coating was allocated 1 point, a dry or medium amount of tongue coating scored 2 points, and a dry or wet tongue with a large amount of coating, or a coating of yellow and black colour scored 3 points. An odorless or flavorful oral cavity odor attracted 1 point, an unpleasant odor attracted 2 points, and a pungent odor scored 3 points. Teeth or dentures without white spots or caries, and a suitable denture scored 1 point, a medium amount of white scale, no caries or interdental pus, or an unsuitable denture scored 2 points, and a large amount of dental calculi, many cavities, cracks, an unsuitable denture, and interdental pus scored 3 points. Moist, soft, lips without cleft were given 1 point, rough, dry lips or those having a few scabs, cleft, or hemorrhagic tendency attracted 2 points, and lips which were dry, were cleft, had many scabs, secretions, and were prone to easy bleeding, were given 3 points. No oral injury or ulcers attracted 1 point, the presence of lip injury scored 2 points, and oral injury scored 3 points. Finally, a wet palate or having a small amount of debris scored 1 point, a dry palate with a small or moderate amount of debris scored 2 points, and a dry palate with a large amount of debris scored 3 points. All items were scored on a scale of 8 to 24, with 8 being a normal oral condition. And the higher the score, the worse the oral cleanliness.
Collected data
Differences in age, gender, educational level, tumor types, tumor stage, radiotherapy, radiotherapy duration, chemotherapy, dentures, smoking history, white blood cell count, oral cleanliness, body mass index, hemoglobin, creatinine, urea nitrogen, blood glucose, vomiting, diarrhea, and other general information were compared between the oral mucositis group and the no oral mucositis group with univariate analysis. The indicators with significant differences in univariate analysis were included in the multivariate logistic regression model, and the independent risk factors affecting oral mucositis in malignant tumor patients were screened out.
Statistical analysis
SPSS 17.0 software was used for statistical analysis, and the enumeration data were displayed by percentage (%). Chi-square test or analysis of variance was used for comparison, and P<0.05 indicated that the difference was statistically significant. Logistic regression analysis was used to analyze risk factors.
Results
Univariate analysis of oral mucositis in patients with malignant tumors (Table 1)
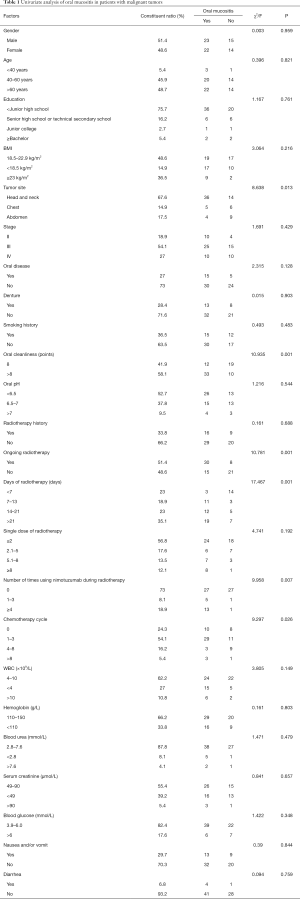
Full table
As can be seen in Table 1, after radiotherapy and chemotherapy in the 74 malignant tumor patients, 45 cases developed oral mucositis of varying degrees, while 29 cases did not, with an incidence of 60.8%. The incidence of oral mucositis in patients with head and neck tumors was significantly higher than that in patients with chest and abdomen tumors and was significantly higher in the group with a high oral cleanliness score than in the group with a low oral cleanliness score. The incidence of oral mucositis in radiotherapy patients was significantly higher than that in non-radiotherapy patients, and the longer the radiotherapy time, the higher the incidence. The incidence of oral mucositis in patients treated with Nituzumab during radiotherapy was significantly higher than that in non-users and significantly higher in patients receiving 1–3 cycles or more than 8 cycles of chemotherapy than that in patients not receiving chemotherapy.
Multivariate logistic regression analysis of oral mucositis in patients with malignant tumors (Table 2)
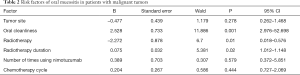
Full table
As can be seen in Table 2, multivariate logistic regression analysis showed that the independent risk factors for oral mucositis in patients with malignant tumors were oral cleanliness, radiotherapy, and radiotherapy duration (P<0.05). Logistic regression analysis included independent variables such as tumor site, oral cleanliness, radiotherapy, radiotherapy duration, times of use of Nimotuzumab, and chemotherapy cycle to clarify the factors that affect the incidence, and the factors excluded by statistical analysis were tumor location, chemotherapy cycle, and times of use of Nimotuzumab.
Discussion
Incidence of oral mucositis in patients with malignant tumors
In this study, 45 of 74 malignant tumor patients developed oral mucositis of varying degrees, with an incidence of 60.8%. According to the study of Çakmak et al. (11) the incidence of oral mucositis in cancer patients undergoing outpatient chemotherapy is 51.7%. This is higher than the 25% seen by Dodd (12), and may be due to the higher proportion of radiotherapy patients in the present study (51.4%). The incidence of oral mucositis was also significantly higher in patients with head and neck tumors (72%) than that in patients with chest and abdomen tumors. Zahn et al. found the incidence of oral mucositis after radiotherapy and chemotherapy for oropharyngeal tumors was as high as 99% (15), which may be because head and neck tumors in that study were not further divided into head tumors and oropharyngeal tumors. The results of these studies show attention should be paid to the oral mucosa of patients with malignant tumors, especially those with head and neck tumors, and timely detection of problems and early intervention should be given to reduce the incidence and degree of oral mucositis. The use of an anti-inflammatory mouthwash can help reduce symptoms of oral mucositis in head and neck tumor patients treated with radiotherapy (16), and in the present study the incidence of oral mucositis was significantly higher in the group with high oral cleanliness score than in the group with low oral cleanliness score. The oral cleanliness score was evaluated from eight aspects: mucous membrane, gums, tongue, odor, teeth, lip, injury, and palate. A higher score indicated worse oral cleanliness, and the more prone the patient was to oral mucositis. Poor oral cleanliness is conducive to the growth and reproduction of bacteria in the oral cavity. Bacteria in the oral cavity play an important role in the occurrence of oral mucositis, and affect its duration and severity (17,18). In clinical work, it is necessary to strengthen oral care, improve oral cleanliness, reduce bacterial reproduction, and reduce the incidence of oral mucositis.
The incidence of oral mucositis in radiotherapy patients was significantly higher than in patients not receiving radiotherapy. The mechanism by which this occurs is the direct cytotoxic effect of radiation, which causes mucosal injury (19). The duration and degree of mucositis induced by radiotherapy are related to the dose, intensity, and cumulative dose of radiotherapy. Therefore, the oral mucosa of patients undergoing radiotherapy, especially when repeated, should be closely observed and early intervention should be given to alleviate the pain of patients. The use of glutamine can reduce the incidence of grade III and IV oral mucositis caused by radiotherapy or chemotherapy (20).
The incidence of oral mucositis in patients who received Nituzumab during radiotherapy was significantly higher than that of non-users, and the greater use of the drug equated with a higher incidence. This may be because the subjects included in this study who received Nituzumab were treated simultaneously with radiotherapy. The first dose was given on the first day of radiotherapy and thereafter once per week of radiotherapy. The longer the duration of radiotherapy, the higher the cumulative radiation dose, and the greater administration of Nituzumab, all equated with a higher the incidence of oral mucositis. Elting et al. suggested that the incidence of oral mucositis in tumor patients receiving targeted therapies such as Bevacizumab, Erlotinib, and Sorafenib was significantly increased (21), which is consistent with the results of the present study.
The incidence of oral mucositis in patients with 1–3 cycles or more than 8 cycles of chemotherapy was significantly higher than that in patients not receiving chemotherapy. According to Jones et al., the incidence of oral mucositis in chemotherapy patients is 20–40%, and the incidence in high-dose chemotherapy patients is 90% (22). Chemotherapy-induced mucositis was related to the dose intensity of the chemotherapeutic drugs, which was consistent with the results of our study. The mechanism of chemotherapy-induced oral mucositis involves anti-tumor drugs leading to the release of inflammatory mediators, which weaken the regeneration ability of epithelial cells causing mucosal injury. Oral cryotherapy can effectively prevent and relieve oral mucositis and anorexia caused by chemotherapy (23).
The key measure for the prevention and treatment of oral mucositis (6) in malignant tumor patients is to maintain the best nutritional support in the process of tumor treatment. If the diet of malignant tumor patients is affected by oral mucositis in the process of treatment, nutritional support programs should be given in time, and analgesic treatment should be given when necessary. At the same time, pay attention to keep your mouth clean. Brush and gargle at least 4 times a day with a soft-bristled toothbrush. When gargling, use non-alcoholic mouthwash with 15 mL mouthwash each time for 1 minute. Avoid eating acrid, coarse, overheated food, lest damage oral mucous membrane.
Risk factors of oral mucositis in patients with malignant tumors
The possible etiology of oral mucositis is caused by drugs, infection and immune deficiency, and the two main causes of oral mucositis in patients with head and neck tumors are high-dose radiotherapy and chemotherapy (6). Mucosal barrier injury can be divided into five stages: initiation, up-regulation of messenger generation, signaling and amplification, inflammatory ulcer formation and final healing (4). In patients receiving radiotherapy, oral mucosal endothelial injury is the early change of mucosal injury, and then due to oxidative stress and reactive oxygen species caused by chemotherapy or radiation therapy, DNA in the upper cortex is damaged, and epithelial cells lose their ability to renew, leading to the death, atrophy and subsequent formation of ulcers of clonal cells (4). Elevated levels of inflammatory factors such as tumor necrosis factor-α (TNF-A) and interleukin-1 and 6 (IL-1 and IL-6) in peripheral blood of patients after chemotherapy may also increase mucosal toxicity (4).
The results of this study showed that oral cleanliness, radiotherapy, and radiotherapy duration were independent risk factors for oral mucositis in patients with malignant tumor. However, there are few studies investigating these factors. Nishii et al. suggested that patients with oropharyngeal cancer, male patients, low hemoglobin levels, low white blood cells or lymphocytes, and cisplatin or cetuximab application were prone to severe oral mucositis (24). However, in the present study, low hemoglobin was not found to be a risk factor, which may be related to the small sample size. Mlak et al. (25) suggested that high concentrations of TNF-α and CC genotypes of TNF-α in blood of patients with head and neck tumor radiotherapy were associated with increased risk of severe oral mucositis. High plasma TNF-α concentration and CC genotype of TNF-α were independent prognostic factors in patients with radiotherapy for head and neck tumors. Çakmak et al. (11) found that advanced age, inappetence and long duration of chemotherapy were risk factors in the study of cancer patients undergoing outpatient chemotherapy. Although Shouval et al. found that age, lower body mass index, smoking, antibiotic use, and methotrexate were associated with the development of moderate to severe oral mucositis (10), their work was on allogeneic hematopoietic stem cell transplant recipients. Age, lower body mass index, and smoking were not found to be risk factors in the present study.
In conclusion, oral cleanliness, radiotherapy, and radiotherapy duration are independent risk factors for oral mucositis in patients with malignant tumors. The results provide a basis for the prevention and intervention of oral mucositis and intervention should be carried out according to the above risk factors. Patients with radiotherapy should be given preventive measures during radiotherapy, and intervention to minimize the incidence and degree of oral mucositis.
Limitations
One limitation of this study is its small sample size, and a larger cohort is recommended to verify the results. After that, the occurrence of oral mucositis of the same tumor or different types of tumor can be analyzed in detail. In addition, we only investigated oral cleanliness, tumor location, radiotherapy, radiotherapy duration, chemotherapy cycle, and the frequency of use of nimotuzumab as risk factors for oral mucositis in patients with malignant tumors. There may be other risk factors such as immune function status not included in this study that could be investigated further in the future.
Acknowledgments
Funding: Nantong Science and Technology Project (Guidance) of Nantong City (MSZ19190), Jiangsu Young Medical Personnel Project (QNRC2016399).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1675
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1675
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1675). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethics board at the Affiliated Hospital of Nantong University and informed consent was taken from all the patients. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013)
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Seiwert TY, Cohen EE. State-of-the-art management of locally advanced head and neck cancer. Br J Cancer 2005;92:1341-8. [Crossref] [PubMed]
- Elad S, Cheng KKF, Lalla RV, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020;126:4423-31. [Crossref] [PubMed]
- Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-Induced mucosal Injury. Cancer 2004;100:1995-2025. [Crossref] [PubMed]
- Elad S, Zadik Y. Chronic oral mucositis after radiotherapy to the head and neck: a new insight. Support Care Cancer 2016;24:4825-30. [Crossref] [PubMed]
- Peterson DE, Boers-Doets CB, Bensadoun RJ, et al. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol 2015;26:v139-51. [Crossref] [PubMed]
- Barkokebas A, Silva IH, de Andrade SC, et al. Impact of oral mucositis on oral-health-related quality of life of patients diagnosed with cancer. J Oral Pathol Med 2015;44:746-51. [Crossref] [PubMed]
- Yarom N, Hovan A, Bossi P, et al. Systematic review of natural and miscellaneous agents for the management of oral mucositis in cancer patients and clinical practice guidelines-part 1: vitamins, minerals, and nutritional supplements. Support Care Cancer 2019;27:3997-4010. [Crossref] [PubMed]
- Pulito C, Cristaudo A, Porta C, et al. Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res 2020;39:210. [Crossref] [PubMed]
- Shouval R, Kouniavski E, Fein J, et al. Risk factors and implications of oral mucositis in recipients of allogeneic hematopoietic stem cell transplantation. Eur J Haematol 2019;103:402-9. [Crossref] [PubMed]
- Çakmak S, Nural N. Incidence of and risk factors for development of oral mucositis in outpatients undergoing cancer chemotherapy. Int J Nurs Pract 2019;25:e12710 [Crossref] [PubMed]
- Dodd MJ, Miaskowski C, Dibble SL, et al. Factors influencing oral mucositis in patients receiving chemotherapy. Cancer Pract 2000;8:291-7. [Crossref] [PubMed]
- Mallick S, Benson R, Rath GK. Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol 2016;273:2285-2293. [Crossref] [PubMed]
- Chalmers JM, King PL, Spencer AJ, et al. The oral health assessment tool--validity and reliability. Aust Dent J 2005;50:191-199. [Crossref] [PubMed]
- Zahn KL, Wong G, Bedrick EJ, et al. Relationship of protein and calorie intake to the severity of oral mucositis in patients with head and neck cancer receiving radiation therapy. Head Neck 2012;34:655-62. [Crossref] [PubMed]
- Konishi M, Verdonschot RG, Shimabukuro K, et al. The effectiveness of mouthwashes in alleviating radiation-induced oral mucositis in head and neck cancer patients: a systematic review. Oral Radiol 2019;35:207-23. [Crossref] [PubMed]
- Sobue T, Bertolini M, Thompson A, et al. Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model. Mol Oral Microbiol 2018;33:212-23. [Crossref] [PubMed]
- Haverman TM, Laheij AMGA, Nie M, et al. Exploring the role of oral microorganisms in the pathogenesis of mucositis by assessing their impact on metabolic activity and reproductive capacity of epithelial cells in vitro. Support Care Cancer 2020;28:4729-35. [Crossref] [PubMed]
- Sonis ST. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol 2009;45:1015-20. [Crossref] [PubMed]
- Peng TR, Lin HH, Yang LJ, et al. Effectiveness of glutamine in the management of oral mucositis in cancer patients: a meta-analysis of randomized controlled trials. Support Care Cancer 2021;29:4885-92. Erratum in: Support Care Cancer 2021 May 13. [Crossref] [PubMed]
- Elting LS, Chang YC, Parelkar P, et al. Risk of oral and gastrointestinal mucosal injury among patients receiving selected targeted agents: a meta-analysis. Support Care Cancer 2013;21:3243-54. [Crossref] [PubMed]
- Jones JA, Avritscher EB, Cooksley CD, et al. Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer 2006;14:505-15. [Crossref] [PubMed]
- Okamoto K, Ninomiya I, Yamaguchi T, et al. Oral cryotherapy for prophylaxis of oral mucositis caused by docetaxel, cisplatin, and fluorouracil chemotherapy for esophageal cancer. Esophagus 2019;16:207-13. [Crossref] [PubMed]
- Nishii M, Soutome S, Kawakita A, et al. Factors associated with severe oral mucositis and candidiasis in patients undergoing radiotherapy for oral and oropharyngeal carcinomas: a retrospective multicenter study of 326 patients. Support Care Cancer 2020;28:1069-75. [Crossref] [PubMed]
- Mlak R, Powrózek T, Brzozowska A, et al. The relationship between TNF-α gene promoter polymorphism (- 1211 T > C), the plasma concentration of TNF-α, and risk of oral mucositis and shortening of overall survival in patients subjected to intensity-modulated radiation therapy due to head and neck cancer. Support Care Cancer 2020;28:531-40. [Crossref] [PubMed]
(English Language Editor: B. Draper)


