
Efficacy and safety of Intense pulsed light therapy for dry eye caused by meibomian gland dysfunction: a randomised trial
Introduction
Meibomian gland dysfunction (MGD) is a chronic, diffuse meibomian gland disease (1,2). The pathogenesis of meibomian gland dysfunction is not fully understood. It may be related to various factors such as abnormal meibomian gland function, eye diseases, environmental factors and so on (3). Abnormal functions of the meibomian glands include insufficient secretion of the meibomian glands, or reduction in the number of congenital meibomian glands or displacement of their openings. The reduction or increase of the meibomian glands can cause eye irritation, abnormal tear film, and inflammation and damage to the ocular surface. Eye diseases include allergic conjunctivitis, meibomian cysts, conjunctival stones, inflammation of the eyelids or cornea, etc., which can cause damage to the cornea and conjunctiva of the eye. During the repair process, scars may be formed on the eyelids, which affects the secretion and excretion of the meibomian glands, aggravates the blockage of the meibomian glands, and causes the dysfunction of the meibomian glands. Environmental factors are mainly bacterial infections causing blockage of the Meibomian glands. The lipids and secretions in the glands will accumulate in the glands. Bacteria adhere to the eyelids and block the catheter. Traditional treatments of dry eye caused by MGD mostly use massage combined with a hot compress to promote the elimination of secretions and dredge the obstruction of the meibomian gland orifice (4,5). However, the effects of this treatment are related to individual operator’s operating experience and proficiency. Additionally, a meibomian gland massage can only relieve temporary clinical symptoms, does not have a long-lasting effect, and can easily lead to the recurrence of dry eye. The vicious circle can cause serious complications, and affect vision. Thus, finding a new and effective treatment for MGD patients with dry eye is an important and difficult problem in the field of ophthalmic diseases.
Intense pulsed light (IPL) was first applied in dermatology and has a good effect in the treatment of skin vasodilation, cavernous hemangioma, and other diseases. Recently, some scholars have proposed that the application of IPL can improve the stability of the tear film and improve the clinical symptoms of patients (6). However, there are few reports on the short- and long-term efficacy and safety of IPL in the treatment of MGD dry eye, and there are few randomized controlled trials of IPL in the treatment of MGD dry eye (7). So, this study sought to compare the effects of IPL treatment and those of the traditional treatment in terms of short- and long-term efficacy, clinical-symptom relief, and the incidence of adverse reactions during treatment in patients with MGD dry eye to provide a scientific theoretical basis for the clinical application and promotion of IPL in the treatment of MGD. We present the following article in accordance with the CONSORT reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1303).
Methods
Research subjects
A total of 132 patients with MGD dry eye, who had been admitted from January 2018 to January 2020 to The Second Hospital of Shanxi Medical University, were selected as the research subjects. To be eligible to participate in the study, patients had to meet the following inclusion criteria: (I) be aged 18 to 80 years old; (II) meet the diagnostic criteria of “eyelid gland dysfunction and dry eye” as per the “expert consensus on clinical diagnosis and treatment of dry eye” formulated by Ophthalmology Society of Chinese Medical Association (8); (III) have a lower lacrimal river height >0.1 mm, and a meibomian gland obstruction level 1
Grouping
In this study, the random number table method was used, 132 random numbers were generated. The random numbers were sorted from small to large. The first 66 patientswere assigned to the test group, and the remaining 66 cases to the control group. Two-parallel method was used in the experiment and allocation ratio is 1:1. There were 122 eyes in the experimental group and 125 eyes in the control group. There was no significant difference between the 2 groups in relation to age, gender, body mass index (BMI), and the course of the disease (P>0.05; see Table 1).

Full table
Interventions
Experimental group
Patients were treated with IPL. The specific steps for this treatment were as follows: each patient assumed the supine position, any glasses were removed, and the facial skin was cleaned. Patients wore goggles on both eyes and closed their eyes. Coupler gel from the tragus was applied to the tragus on the other side to fill the facial skin. The operator also wore goggles and used the OPT system of the RH-I1504005 light pulse dry eye treatment instrument produced by Shanxi Ruihao Biotechnology Co., Ltd. to administer the treatment. The pulse wavelength was set to 590 nm, and the energy parameters were based on each patient’s tolerance, the skin condition was adjusted within 10
Control group
Patients were treated with a meibomian gland massage combined with a hot compress treatment. The specific steps were as follows: a hot compress was applied to the affected eye of the patient for about 15 min. An experienced ophthalmologist used cotton swabs to assist the meibomian gland pad to massage the upper and lower meibomian glands to promote secretion elimination.
Therapeutic effects
The following 4 indicators were used to judge the treatment effects: eye redness, the number of meibomian glands, the tear film rupture time, and corneal fluorescence staining. The evaluation was undertaken on the 30th day after treatment. The treatment effect was classified as significant if the symptoms had disappeared, the red eye rating was 0, the meibomian gland number score was 0, the tear film rupture time was more than 10 s or more than 5 s, the added value was more than 2 s, and the corneal fluorescein staining score was 0. The treatment was classified as effective if the patient experienced symptom relief, the redness rating was lower than that before treatment, the meibomian gland number score was lower than that before treatment, or the tear film rupture time was longer than that before treatment, or the corneal fluorescein staining score was lower than that before treatment. The effect was classified as invalid if there was no improvement in symptoms, the red eye rating had not changed from that before treatment, the number of meibomian glands had not decreased from that before treatment, the tear film rupture time had not changed from that before treatment, and the corneal fluorescein staining score was not lower than that before treatment. Total effective rate (%)=(significant effective + effective)/total number of affected eyes ×100%. Therapeutic effects are primary endpoint. Improved clinical features, safety analysis and patient satisfaction evaluation are secondary endpoint.
Improved clinical features
The meibomian gland number score, lacrimal river height measurement and Schirmer test were performed before treatment, 7 days after treatment, and 30 days after treatment. The evaluation criteria were as follows:
- The meibomian glands score: 0 points were assigned if the 5 glands in the center of the meibomian were normal, 1 point was assigned if 1 or 2 glands without secretions were mildly abnormal; 2 points were assigned if only 1 or 2 glands discharged secretions with moderate abnormalities; and 3 pointswere assigned if there were 5 glands without secretions for severe abnormalities. The meibomian gland scores for moderate and severe abnormalities suggested the possibility of dry eye.
- The height measurement of the tear river. The tear river refers to the long strip of tear river between the upper and lower eyelid edges. This height may be normal or abnormal. A normal height ranges from 0.4–1.0 mm. If the height is less than 0.35 mm, dry eye may occur in the eye.
- The lacrimal gland secretion function. The Schirmer test (9) was used to evaluate the secretion function of the lacrimal gland. Two filter papers (5 mm ×35 mm) were used and placed at the junction of the palpebral fissure internal 1/3 and middle 1/3. The wet length of the filter paper was checked after the eyes had been closed for 5 minutes. If the wet length is lower than 5 mm, tear secretion will decrease.
Safety analysis
Following treatment, patients were evaluated at 7 and 30 days after treatment to observe the lens condition and any intraocular pressure changes. The presence of cataract or abnormal intraocular pressure was identified as an adverse event. Incidence of adverse events (%) = number of adverse event cases/total number of cases in this group ×100%.
Patient satisfaction evaluation
A self-made satisfaction questionnaire was used to investigate patients’ levels of satisfaction with the treatments. The satisfaction evaluation was carried out according to treatment methods and treatment effects. It was divided into satisfaction, general satisfaction and dissatisfaction. Total satisfaction rate (%) = (satisfaction + general satisfaction)/total number of cases in this group ×100%.
Statistical analysis
Statistical software R4.0.3 was used to compare the significance values of the results of the experimental and control groups. The continuity variables are represented by the normal distribution (mean ± standard deviation); a t-test was used for the analysis. The non-normal distribution is represented by M (p25–p75); a rank sum test was used for the analysis. The categorical variables are represented by [n (%)]; the χ2 test was used for the analysis, the Fisher exact probability method was used for T<1, and the rank sum test was used for individual ordered data. Repeated measurement data were analyzed using generalized estimation equations. Statements of significance were based on P values of less than 0.05.
Results
Comparison of the therapeutic effects between the 2 groups of patients
A total of 132 patients with dry eye caused by MGD admitted to the Second Hospital of Shanxi Medical University between January 2018 and January 2020 were followed up for 30 days. Baseline characteristics of patients is shown in Table 1. The total effective rates of the experimental group and the control group were 90.2% and 80.0% respectively. The difference in the effective rate (10.2%) was statistically significant (P<0.05). The total effective rate of the experimental group was equivalent to that of the control group (see Table 2). The participant flow is shown in Figure 1.

Full table
Comparison of meibomian gland quality scores between the 2 groups before and after treatment
The number of cases with a meibomian gland quality score of 2–3 before treatment, 7 days after treatment, and 30 days after treatment in the experimental group was 72 (59.0%), 38 (31.1%), and 24 (19.7%), respectively. In the control group, the number of eyes with a meibomian gland quality score of 2–3 before treatment, 7 days after treatment, and 30 days after treatment was 77 (61.6%), 70 (56.0%), and 65 (52.0%), respectively. There was a significant difference in the eye rate of the meibomian gland quality score of 2
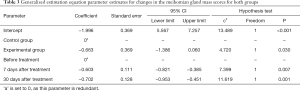
Full table
Comparison of the height of the tear river between the 2 groups before and after treatment
The numbers of eyes with a lacrimal river height ≤0.35 mm before treatment, 7 days after treatment, and 30 days after treatment in the experimental group were 88 (72.1%), 58 (47.5%), and 45 (36.6%), respectively. The eye rates of the control group before treatment, 7 days after treatment, and 30 days after treatment were 79 (75.2%), 65 (52.0%), and 78 (63.4%), respectively. There was no significant difference in the eye rates between the different groups of patients with a tear river height ≤0.35 mm (P>0.05). The difference in the eye rate of a tear river height ≤0.35 mm measured at different times was statistically significant (P<0.05). With the extension of time, the eye rate of a tear river height ≤0.35 mm decreased gradually. Compared to before treatment, the eye rate of patients with a lacrimal river height ≤0.35 mm at 7 and 30 days after treatment was lower than that before treatment (see Table 4).
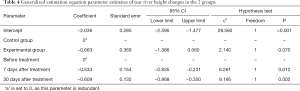
Full table
Comparison of the tear secretion Schirmer test results between the 2 groups before and after treatment
The rates of tear secretion ≤5 mm in the experimental group before treatment, 7 days after treatment, and 30 daysafter treatment were 65 (53.3%), 48 (39.3%), and 27 (22.1%), respectively. In the control group, the rates of tear secretion ≤5 mm before treatment, 7 days after treatment, and 30 days after treatment were 57 (45.6%), 57 (45.6%), and 43 (34.4%), respectively. The results of the generalized estimation equation analysis are set out in Table 5. The difference in the rates of tear secretion ≤5 mm between the 2 groups was statistically significant (P<0.05), and the rate of tear secretion ≤5 mm in the experimental group was lower than that in the control group. The difference in rates of tear secretion ≤5 mm measured at different times was statistically significant (P<0.05). With the extension of time, the rate of tear secretion ≤5 mm decreased gradually. Compared to before treatment, the rate of tear secretion ≤5 mm in the 30-day after-treatment group was lower than that before treatment; however, the rate of tear secretion ≤5 mm in the 7-day after-treatment group was not statistically significant to that before treatment (see Table 5).
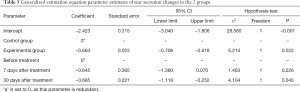
Full table
Comparison of treatment safety between the 2 groups
No adverse reactions were observed in the 2 groups during treatment; the difference was not statistically significant (P>0.05).
Comparison of treatment satisfaction between the 2 groups
After 7 days of treatment, the total satisfaction levels of the experimental group and control group were 86.4% and 66.7% respectively; the difference between the 2 groups was statistically significant (χ2=7.125, P=0.018 <0.05); After 30 days of treatment, the total satisfaction levels of the experimental group and control group were 92.4% and 71.2% respectively; the difference between the 2 groups was statistically significant (χ2=9.981, P<0.01). The total satisfaction of the experimental group was higher than that of the control group at 7 days and 30 days after treatment.
Discussion
MGD dry eye is mainly related to microbial infection, inflammation, and lipid deficiency (10). The traditional clinical treatment method of eye meibomian gland massage can promote the discharge of meibomian gland secretions through mechanical action, relieve the obstruction of meibomian gland, and improve the function of the eyelid gland; however, the effects are not ideal (11). Previous studies have found that only 7% of patients can completely tolerate eyelid obstruction and discharge secretions. Toyos proposed IPL as a new treatment for MGD-related dry eye. IPL is a type of strong composite light with a high intensity, wide wavelength, and continuity, and is non-coherence generated. The light is emitted by a flash lamp (wavelength of 500
In this study, the total effective rates of experimental group and control group were 90.2% and 80.0% respectively, and the therapeutic effects of experimental group was better than that of control group (P<0.05). The generalized estimation equation was used to analyze the longitudinal data and construct a relevant statistical model, we found that with the extension of time, patients’ meibomian gland quality score was severe, and the rate of a tear river height ≤0.35 mm and a tear secretion ≤5 mm decreased (P<0.05). Further, The incidence of abnormal blepharian gland quality score, lacrimal river height ≤0.35 mm, and tear secretion ≤5 mm in IPL treatment group were lower than those in hot compress group (P<0.05). Consistent with the findings of Fang et al. (15) and Rong et al. (16), IPL treatment was not worse than the traditional treatment. Indeed, in relation to the secondary efficacy indicators, IPL therapy was shown to improve the clinical symptoms of MGD-related dry eye. The light of IPL can be preferentially and selectively absorbed by oxygen and hemoglobin in abnormal blood vessels, and converted into heat energy to increase temperature in tissues (17,18). After heating up, the damage threshold of blood vessels can be reached, and abnormal blood vessels can then be destroyed, which in turn results in occlusion degeneration, and the gradual replacement of microscopic tissues, and achieves the purpose of treating MGD dry eye (19). Additionally, the destruction of abnormal blood vessels also reduces the release and conduction of inflammatory mediators and promotes the recovery of normal function of meibomian glands (20,21).
In the safety analysis, no adverse events were observed during and after treatment in the 2 groups of patients. Thus, IPL treatment or a traditional meibomian gland massage combined with a hot compress treatment appears to be safer than the methods used in the study of Yang et al. (22). The significant decrease in the incidence of adverse events in this study may be related to the mild degree of inflammation and the optimal development of IPL technology in the MGD-related dry eye patients included in this study.
In the satisfaction survey, patients were more satisfied with IPL treatment at 7 days and 30 days after treatment than the treatment of a meibomian gland massage combined with a hot compress (P<0.05). Thus, the effects, operation, and acceptance of IPL in the treatment of MGD-related dry eye were high among patients, and it has certain clinical feasibility.
This study had a number of limitations. The sample size was small and the case source was single (i.e., the patients came from one hospital only). Thus, there may be a selection bias. There was also a lack of in-depth research on the molecular mechanism of IPL in the treatment of MGD-related dry eye disease. Some patients had to use antibiotic eye drops to resist inflammation and infection; however, the effects of antibiotic tumor or inflammatory factors on the treatment effects was not considered. The follow-up time was also too short. Future studies should follow-up with patients for 2 to 12 months after IPL treatment. Finally, the severity of MGD-related dry eye was not corrected, and the reliability of the results are limited. All these issues could be addressed by the subsequent expansion of the sample size, conducting a multi-center study, and undertaking in-depth research.
Conclusions
In summary, the curative effects of IPL treatment of MGD-related dry eye were not worse than those of the traditional meibomian gland massage combined with hot compress. IPL can improve MGD-related dry eye in patients with eye symptoms, is safe, is highly accepted among patients, and could be applied in clinical settings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1303
Trial Protocol: Available at https://dx.doi.org/10.21037/apm-21-1303
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1303
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1303). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of The Second Hospital of Shanxi Medical University and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- . Systematic review and meta-analysis of treating meibomian gland dysfunction with azithromycin. Eye (Lond) 2020;34:1797-808. [Crossref] [PubMed]
- , et al. Intense Pulsed Light Therapy for Patients with Meibomian Gland Dysfunction and Ocular Demodex Infestation. Curr Med Sci 2019;39:800-9. [Crossref] [PubMed]
- , et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol 2016;100:300-6. [Crossref] [PubMed]
- , et al. Meibomian gland dysfunction and dry eye: Diagnosis and treatment Orv Hetil 2021;162:43-51. [PubMed]
- , et al. Prevalence of Meibomian Gland Dysfunction and Its Effect on Quality of Life and Ocular Discomfort in Patients with Prosthetic Eyes. Prosthesis 2020;2:91-9. [Crossref]
- , et al. Intense Pulsed Light Therapy with Meibomian Gland Expression for Dry Eye Disease. Can J Ophthalmol 2020;55:189-98. [Crossref] [PubMed]
- Vigo L, Giannaccare G, Sebastiani S, et al. Intense Pulsed Light for the Treatment of Dry Eye Owing to Meibomian Gland Dysfunction. J Vis Exp 2019;(146).
- Ophthalmology Branch of Chinese Medical Association. Expert Consensus (2013) on Clinical Diagnosis and Treatment of Dry Eye. Chinese Community Doctors 2013;2013:11-2.
- , et al. Study of Artificial Tear Combined With Flumirone in the Treatment of Dry Eye With Tarsal Gland Dysfunction. China Health Standard Management 2019;10:54-6.
- , et al. Effectiveness of intense pulsed light in the treatment of meibomian gland dysfunction. Chinese Journal of Experimental Ophthalmology 2019;37:185-9.
- , et al. Clinical Observation on Safety and Efficacy of Intense Pulsed Light Wave in Treating Dry Eye Related to Meibomian Gland Dysfunction. Guide of China Medicine 2019;17:163-4.
- , et al. Clinical study of intense pulsed light in the treatment of dry eye with meibomian gland dysfunction. Shanxi Medical Journal 2018;47:81-3.
- . Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci 2015;56:1965-70. [Crossref] [PubMed]
- , et al. The Complex Treatment Algorithm for Dry Eye Syndrome with Meibomian Gland Dysfunction. Combination of Intense Pulsed Light (IPL) with Eyelid Hygiene and Artificial Tears. Ophthalmology in Russia 2020;17:640-7. [Crossref]
- , et al. Clinical analysis of intense pulsed laser combined with meibomian gland massage for dry eye associated with meibomian gland dysfunction. Medical Journal of Wuhan University 2020;41:315-8.
- , et al. Evaluation of short-term effect of intense pulsed light combined with meibomian gland expression in the treatment of meibomian gland dysfunction. Chinese Journal of Ophthalmology 2017;53:675-81. [PubMed]
- . Observation on the clinical effect of intense pulsed light combined with local massage in the treatment of xerophthalmia caused by meibomian gland dysfunction. China Practical Medical 2020;15:31-4.
- , et al. Clinical observation of intense pulsed light therapy for meibomian gland dysfunction. International Eye Science 2017;10:162-5.
- , et al. The role of intense pulsed light (IPL) in the treatment of meibomian gland dysfunction (MGD). European Journal of Plastic Surgery 2019;42:563-8. [Crossref]
- . Effects of meibomian gland massage in the treatment of xerophthalmia with meibomian gland dysfunction. China Medicine and Pharmacy 2020;10:246-8.
- . Emerging strategies for the treatment of Meibomian gland dysfunction. Chinese Journal of Ophthalmology 2019;55:465-8. [PubMed]
- , et al. Safety and Efficacy of Intense Pulsed Light in the Treatment of Meibomian Gland Dysfunction. Advances in Clinical Medicine 2020;10:755-60. [Crossref]
(English Language Editor: L. Huleatt)



