
Small cell metastatic prostate cancer with ectopic adrenocorticotropic hormone hypersecretion: a case report
Introduction
Extrapulmonary small cell carcinoma is uncommon, nevertheless, one of the most frequently affected organs is prostate, in about 10% of cases (1). Its diagnosis should be considered in patients with prostate cancer symptoms and relative low prostate specific antigen (PSA) level. This tumor is very aggressive due to its high rate of distant dissemination with visceral metastases at diagnosis. We report the case of a metastatic oat-cell prostate cancer with adrenocorticotropic hormone (ACTH) hypersecretion and an unfavorable clinical course.
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-656).
Case presentation
We report the case of a 65-year-old patient who attended the emergency department with one-day acute urinary retention, arterial hypertension, and edema in lower extremities. In addition, he presented two months symptoms of dysuria, frequent urination and incontinence with no constitutional syndrome. Blood test and gas analysis showed hypokalemia of 2.4 mEq/L and metabolic alkalosis. Abdomino-pelvic ultrasound demonstrated prostate hypertrophy grade IV/IV (80 g) with poorly defined margins and perivesical lymph node formations. Given the hyperaldosteronism symptoms and ultrasound results, he underwent hospital’s admission for additional testing and treatment.
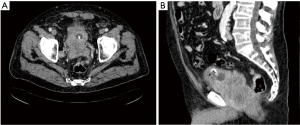
Initial treatment consisted of saline solution and potassium chloride infusion. A thoracoabdominopelvic computed tomography (TAP-CT) was performed showing a prostatic mass of approximately 10 cm with extracapsular extension and multiple metastatic pelvic adenopathies without visceral or metastatic bone involvement (Figure 1). Digital rectal examination detected an abnormal gland and a transrectal prostate biopsy was performed. Histology revealed small cells with large areas of necrosis, indicative of undifferentiated small cell carcinoma. immunohistochemistry (IHC) analysis reported “dot” shape cytokeratin AE1/AE3 positivity, CD56 and focal positivity for synaptophysin, being chromogranin and ACTH negative. Hormone blood test showed PSA level of 2.45 ng/mL, cortisol (COR) 288 µg/dL, ACTH 479 pg/mL,aldosterone (ALD) 102 pmol/L, and specific neuronal enolase (NSE) 35.60 ng/mL.
Patient presented persistent diarrhea due to rectal invasion, psychosis, and steroid myopathy. Given the poor response of hypercortisolemia to ketoconazole, in the context of paraneoplastic syndrome with ACTH hypersecretion, he was transferred to the Critical Care Resuscitation Unit, to begin with continuous intravenous infusion of etomidate. Twenty-four hours after admission, he presented severe respiratory failure with evidence of pneumonia that led to endotracheal intubation, invasive mechanical ventilation and administration of vasoactive drugs. Furthermore, he suffered from atrial fibrillation which could be successfully reversed with electrical cardioversion and amiodarone. Twenty-one days after admission, patient’s condition worsened developing septic shock with multiple organ failure and cardiac arrest.
The authors have obtained the informed consent from the patient’s family due to the patient’s death in the Critical Care Resuscitation Unit. All procedures were followed in accordance with the ethical standards of the Human Research Ethics Committees (HREC) and the Declaration of Helsinki revised in 2013.
Discussion
Small cell prostate cancer (SCPC) represents a minority of 0.5–2% of all prostate cancers (1-4). In 1977, Wenk et al. described the first case of SCPC (1). There is no consensus about its histogenesis, although neuroendocrine (NE) tumors are believed to be derived from totipotent stem cells. NE tumors are classified into three groups: 50% are pure NE tumors, 20% are mixed adenocarcinoma and NE and 30% are adenocarcinomas dedifferentiated to NE tumors during Androgen Deprivation Therapy (ADT) (2).
The average age of SCPC patients is 65 years. It is characterized by a local and distant rapid progression with visceral and osteolytic bone metastases, with low or slightly elevated PSA levels, unlike adenocarcinoma (1-4). Moreover, it can present as paraneoplastic syndromes including Cushing’s syndrome or hypercalcemia, which worsens prognosis (1). As this tumor can go unnoticed, a high diagnostic suspicion is necessary, being the biopsy the gold standard for its confirmation. The anatomopathological result reveals small cells with a high nuclear: cytoplasmic ratio; absent or discrete nucleoli; hyperchromatic core and increased mitotic rates and necrosis (3). IHC analysis should include prostatic [PSA, prostatic specific acid phosphatase (PSAP), prostate-specific membrane antigen (PSMA), ps501] and NE markers (CD56, TTF-1, synaptophysin, and chromogranin). In SCPC, the former will be practically normal while in poorly differentiated adenocarcinomas they will be slightly elevated (3).
Given the low incidence, there is a lack of standardization and evidence-based medicine in SCPC management. Prostatectomy or radiation therapy is suggested in local-early-stage tumors (3); however, if tumor is locally advanced, systemic chemotherapy with a platinum-based agent combined with etoposide is the mainstay of therapy (1-4). Palliative radiotherapy and other supportive care can be considered in some cases (3). Furthermore, it does not respond to ADT because of its few androgen receptors (1-4), so it would only be a valid treatment in mixed tumors. As pulmonary and extrapulmonary small cell carcinomas share histological characteristics, the same treatment target could be used in both cases. This is why second-line therapies are being investigated, such as membrane receptor tyrosine kinases inhibitors (c-KIT); a toxic immunoconjugate B3-10901 to membrane glycoprotein CD56; membrane glycolipids vaccines; GRP receptor antagonists; mTOR inhibitors; anti-PD-1; anti-EGFR and anti-angiogenic drugs or relaxin receptor RXFP1 suppressors (1,3).
SCPC is an aggressive tumor that is diagnosed in advanced stages. Median survival at diagnosis is 10 months (1,4) and less than 5% of patients survive 2 years (1).
Conclusions
SCPC is a rare type of tumor, no standard therapeutic regimen exists, and prognosis is poor due to its local and distant aggressiveness. A high diagnostic suspicion is necessary, especially in patients with low PSA levels, lack of response to ADT, visceral metastases at diagnosis and paraneoplastic syndromes, which is a poor prognostic factor.
Pathological study with IHC analysis, including NE markers, are essential for its diagnosis.
New lines of treatment are being investigated.
Acknowledgments
The authors thank the patient’s family for allowing them to publish the case report and the images taken. They would also like to extend their gratitude to the Departments of Urology, Radiology, Endocrinology, Anesthesiology and Reanimation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-656
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-656). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures were followed in accordance with the ethical standards of the Human Research Ethics Committees (“HREC”) and the Declaration of Helsinki revised in 2013. The authors have obtained the informed consent from the patient’s family due to the patient’s death in the Critical Care Resuscitation Unit. The protocol of their hospital on the publication of patient data has been followed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alves D, Calmeiro ME, Silva R, et al. Small-cell neuroendocrine cancer of the prostate: an atypical presentation of a common disease. BMJ Case Rep 2016;2016:bcr2016216199. [Crossref] [PubMed]
- Maebayashi T, Abe K, Aizawa T, et al. Solitary pulmonary metastasis from prostate cancer with neuroendocrine differentiation: a case report and review of relevant cases from the literature. World J Surg Oncol 2015;13:173. [Crossref] [PubMed]
- Wood A, Adra N, Cheng L, et al. Metastatic small cell carcinoma of the prostate in an octogenarian with significant response to first-line chemotherapy. Hematol Med Oncol 2017;2:1-3. [Crossref]
- Zaffuto E, Pompe R, Zanaty M, et al. Contemporary Incidence and Cancer Control Outcomes of Primary Neuroendocrine Prostate Cancer: A SEER Database Analysis. Clin Genitourin Cancer 2017;15:e793-800. [Crossref] [PubMed]


