
Comparison of HBV-DNA and HBeAg as antiviral therapeutic indicators among HBV-infected pregnant women: a systematic review and meta-analysis
Introduction
Hepatitis B virus (HBV) infection is an important issue endangering human health worldwide. In 2015, the global prevalence of chronic HBV was 3.5%, 257 million people were chronically infected with HBV all over the world, between 1990 and 2015, the global incidence of liver cancer increased by 75%, of which 42% were HBV-related hepatocellular carcinoma (HCC) (1), and the majority of them lived in Western Pacific and Africa regions assessed by World Health Organization (WHO), patients in these areas have limited medical resources.
In 2016, WHO proposed the goal of “eliminating hepatitis B” by 2030, aiming to reduce HBV incidence by 90% and mortality by 65% (2). The development of chronic hepatitis B is closely related to the age of hepatitis B virus infection, infected with hepatitis B before one year old, nearly 90% will develop chronic infection (3-5). Therefore, preventing mother-to-child transmission (MTCT) of hepatitis B is an important measure to achieve WHO’s goals.
In order to prevent MTCT, WHO recommend that children born to mothers infected with hepatitis B be vaccinated within 24 hours of birth (6). However, even if the newborn is immunized with hepatitis B vaccine and hepatitis B immunoglobulin in time after birth, there are still cases of mother-to-child transmission, and these transmissions are often related to mothers’ high viral load. Pregnant women with high viraemia (≥5.3 log10IU/mL) are suggested to be given antiviral treatment in the second or third trimester of pregnancy to reduce the HBV-DNA load before delivery, and the risk of MTCT will be greatly reduced (7-9). The implementation of antiviral treatments in pregnant women with high viral loads to reduce mother-to-child transmission has set a test for pregnant women to screen for Hepatitis B virus surface antigen (HBsAg) and HBV-DNA, WHO recommends perinatal screening for HBsAg and subsequent HBV-DNA testing to confirm whether pregnant women need antiviral prophylaxis (10).
However, in the Western Pacific and African countries, resources for HBV-DNA screening of pregnant women with hepatitis B are limited. HBV-DNA is usually detected by real-time fluorescent quantitative PCR. This method requires high detection cost and high technical abilities and capabilities of the laboratories (11). Moreover, the implementation status of HBV-DNA testing around the world is still unclear, but there must be a gap in the implementation rate between regions. Alternatively, hepatitis B e antigen (HBeAg) positive is considered to be a manifestation of a high replication state of hepatitis B virus (12), the detection of which is cheaper and the same blood sample that was used to screen for HBsAg can be used in the detection. Therefore, detection of HBeAg is more common and acceptable than HBV-DNA in Western Pacific and Asian countries.
This systematic review and meta-analysis aim to find out the implementation status of global HBV-DNA testing, the distribution of HBV-DNA levels and hepatitis B infection rate of newborns born to mothers with different HBeAg states.
We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1560).
Methods
Study inclusion and procedures
For this systematic review and meta-analysis, we searched five electronic databases including PubMed, Embase, Cochrane Library, Scopus, and China National Knowledge Infrastructure (CNKI) for studies published between Jan 1, 2000, and Nov 16, 2020. Any original research, including conference abstracts will be considered. Three terms, “HBV”, “DNA level”, and “pregnancy” and their variations were used in the search strategy (see https://cdn.amegroups.cn/static/public/apm-21-1560-1.doc, for the full search strategy). For the question, what’s the rate of implementation of HBV-DNA testing, we included studies on the number of pregnant women with hepatitis B undergoing HBV-DNA testing during pregnancy. Studies with a sample size of less than 30 and studies on the impact of certain management methods on the implementation of HBV-DNA testing were excluded for representativeness and accuracy of data. For the question, how is HBV-DNA level distributed under different statuses of HBeAg, we included studies that reported viral load categories by HBeAg status within ranges of ≥5.3 log10IU/mL, <4.3, 4.3–5.29, 5.3–6.29, 6.3–7.29, ≥7.3 log10IU/mL, or ranges of ≥5 log10IU/mL, <4, 4–4.99, 5–5.99, 6–6.99, ≥7 log10IU/mL for the transmission rates varies with these categories and the treatment indication is different. Studies with a sample size of less than 30 and that selected pregnant women on the basis of HBV DNA levels or studies on antiviral treatment during pregnancy were excluded due to the possibility of affecting the natural distribution of HBV DNA. For the question, what’s the MTCT rate by HBeAg status, we included studies with rate of infants’ infection by mothers’ HBeAg statuses, which was defined as HBsAg or HBV DNA positive in infants assessed at 6–12 months of age after three or more doses of hepatitis B vaccine were completed. Studies with a sample size of infants less than 10 or mothers received antiviral therapy during pregnancy were excluded as this will have an impact on the MTCT rates. Further, in order to avoid the influence of different viral infections on the level of hepatitis B virus, we excluded the studies of HIV and HCV co-infection. Duplicate studies were also excluded.
Data extraction
Data were extracted by two researchers independently using the pre-designed tables. If there is any hesitation in the data extraction process, all scholars in this study will evaluate the literature to ensure the accuracy of the data (see the https://cdn.amegroups.cn/static/public/apm-21-1560-1.doc, for details of data extraction). The results of HBV-DNA in copies/mL are all divided by 5 and converted into results in IU/mL (13). The methodological quality of the studies included was assessed using an 11-item checklist (https://cdn.amegroups.cn/static/public/apm-21-1560-1.doc) which was recommended by Agency for Healthcare Research and Quality (AHRQ). An item would be scored ‘0’ if it was answered ‘NO’ or ‘UNCLEAR’; if it was answered ‘YES’, then the item scored ‘1’. Article quality was assessed as follows: low quality =0–3; moderate quality =4–7; high quality =8–11.
Statistical analysis
We pooled the implementation rate of HBV-DNA testing, probability of each maternal viral load category by HBeAg statuses and rate of mother-to-child transmission using random-effects model when I2≥50% and fixed-effects model when I2<50% after data was made a Freeman-Tukey double arcsine transformation. RRs (relative risks) with 95% confidence intervals were pooled using fixed-effects model.
The protocol was registered in PROSPERO, CRD42021235711.
Results
Study characteristics
A total of 9,575 records were identified from databases we searched, of which 537 articles were assessed in depth, 79 articles met the inclusion criteria finally (Figure 1). The characteristics of articles are presented in the https://cdn.amegroups.cn/static/public/apm-21-1560-1.doc. Of the 79 articles included, 26 studies involving 8 countries and regions reported the implementation rate of HBV-DNA and 57 studies reported the HBV-DNA level by HBeAg status, 11 studies reported the cases of mother-to-child transmission by mothers’ HBeAg status. Most of these studies (n=62, 78.48%) were done in the countries in the WHO Western Pacific Region. Forty three of 57 studies (75.44%) which reported the HBV-DNA level by HBeAg status also reported the assay to detect HBeAg, enzyme immunoassay was the most frequently used method (n=32, 74.41%), followed by chemiluminescent immunoassay (n=9, 20.93%), fluorescent immunoassay (n=1, 2.33%), and rapid diagnostic tests (n=1, 2.33%). Forty-four (77.19%) reported the assay to detect HBV-DNA, all studies used PCR to quantify HBV-DNA.
The risk of bias assessment and publication bias are summarized in the https://cdn.amegroups.cn/static/public/apm-21-1560-1.doc, we found no publications bias in this systematic review and meta-analysis.
The implementation rate of HBV-DNA testing
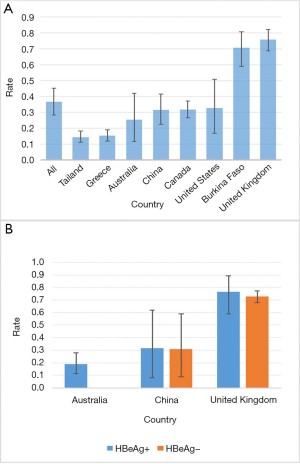
For the implementation rate of HBV-DNA testing, we assessed 28,649 pregnant women with positive HBsAg from 26 studies (https://cdn.amegroups.cn/static/public/apm-21-1560-1.doc). Overall, the implementation of HBV-DNA testing is relatively low globally, with rate of 36.6% (95% CI, 28.3–45.3%) (Figure 2A). These studies involved a total of 8 countries and regions, of which Greece and Thailand had the lowest detection rates, less than 20% of pregnant women with hepatitis B underwent HBV-DNA testing. Nine studies were conducted in China, a total of 9,842 pregnant women with hepatitis B were included, and the pooled detection rate was 31.6% (95% CI, 22.4–41.5%). Three studies were conducted in the UK and included a total of 654 pregnant women with hepatitis B. The HBV-DNA detection implementation rate was 75.9% (95% CI, 68.8–82.3%), the highest among all countries. Only studies in Australia, China and the United Kingdom reported the detection of HBV-DNA based on HBeAg status (Figure 2B), pregnant women with positive and negative HBeAg had almost the same probability of being tested for HBV-DNA, which was 31.7% (95% CI, 8.0–61.9%) vs. 31.1% (95% CI, 9.0–59.1%) in China and 76.5% (95% CI, 58.8–89.3%) vs. 72.8% (95% CI, 67.9–77.2%) in the UK.
HBV-DNA level distribution by mothers’ HBeAg statuses
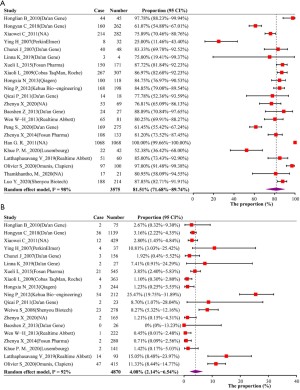
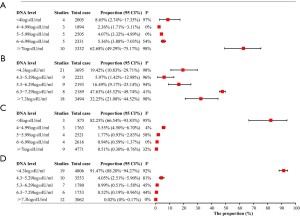
From the previous results, we knew that a considerable number of pregnant women with hepatitis B had not been tested for HBV-DNA, we then explored the distribution of HBV-DNA levels of pregnant women in different states of HBeAg. First, we explored the positive rate of HBeAg among HBsAg-positive pregnant women, 60 articles involving 30,386 research subjects reported this rate, the pooled rate was 31.27% (95% CI, 28.49–34.12%). In this part of the study, HBV-DNA classification data by HBeAg status were available in 57 articles. First, we confirmed the probability that when a pregnant woman is HBeAg positive, her HBV-DNA will reach the recommended treatment threshold for antiviral during pregnancy (≥5.3 log10IU/mL), the probability was 81.51% (95% CI, 71.68–89.74%) (Figure 3A), and which was 4.08% (95% CI, 2.14–6.54%) in the HBeAg negative group (Figure 3B). Due to the differences in the HBV-DNA classification methods reported in studies, in order to clarify the risk as fully as possible, we also explored the probability of HBV-DNA ≥5 log10IU/mL by HBeAg status, which was 85.50% (95% CI, 81.37–89.22%) when HBeAg is positive and 6.33% (95% CI, 3.92–9.24%) when HBeAg is negative. Since the risk of mother-to-child transmission of hepatitis B is positively correlated with the level of HBV-DNA (14), we have also studied the distribution of HBV-DNA levels under different conditions of HBeAg. More than 60% of HBeAg-positive pregnant women have HBV-DNA levels of 6log10IU/mL or higher (Figure 4A,4B). In HBeAg-negative pregnant women, there was an 82.25% (95% CI, 66.54–93.83%) probability that the HBV-DNA level will be lower than 4 log10IU/ml, and a 91.47% (95% CI, 88.20–94.27%) probability that it will be lower than 4.3 log10IU/mL (Figure 4C,4D).
MTCT rate by mothers’ HBeAg statuses
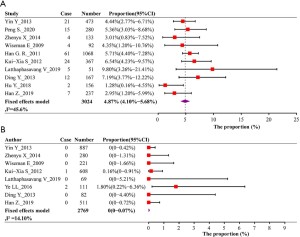
We further analyzed the infection risk of newborns when their mothers with different HBeAg status didn’t received antiviral treatment. All articles documented the immunization strategy for newborns, and had all received the immunization procedures stipulated by various national policies. All of the 5,793 babies received at least 3 injections of hepatitis B vaccine. Except for the 120 newborns in the Latthaphasavang’s study (15) that were not be recorded whether they were vaccinated with hepatitis B immunoglobulin, other children were also vaccinated with HBIG within 24 hours of birth. Among 3,024 infants from HBeAg positive pregnant women in 10 studies, the pooled mother-to-child transmission rate was 4.87% (95% CI, 4.10–5.68%) (Figure 5A), and among 2,769 babies from HBeAg negative mothers in 8 studies, the pooled MTCT rate was 0 (95% CI, 0–0.07%) (Figure 5B). We also evaluated the RR of mother-to-child transmission in mothers with different statuses of HBeAg, 7 studies reported MTCT rate simultaneously when HBeAg is positive or negative. Compared to the mother with HBeAg-negative, the RR of transmission was 30.40 (95% CI, 11.31–81.72) for the mother with HBeAg-positive (https://cdn.amegroups.cn/static/public/apm-21-1560-1.doc).
Discussion
In this study, we first found that the implementation rate of HBV-DNA testing in pregnant women with hepatitis B is very low globally. If measures were not taken to increase the detection rate of HBV-DNA in pregnant women, or other reference indicator replacing HBV-DNA for pregnancy treatment were not found, some children will still suffer MTCT (16), even if newborns received standard vaccination. In 2015, the global prevalence of chronic HBV was 3.5% (1), which means about 35,000 of every 1 million pregnant women are chronically infected with hepatitis B, as we reported above, the HBV-DNA testing rate was 36.6% globally, then about 22,204 mothers will miss the HBV-DNA test, of which about 6,943 pregnant women are HBeAg positive, if they do not receive antiviral treatment during pregnancy, it will cause about 338 infants to be infected.
In order to explore the effectiveness of HBeAg instead of HBV-DNA as a therapeutic indicator, we analyzed the distribution of HBV-DNA in pregnant women with different statuses of HBeAg, and found that when HBeAg is positive, the pregnant women’s HBV-DNA is highly likely to be high load, and the tranmission rate among those HBeAg-positive women is 4.87%. Among HBeAg-negative pregnant women, although about 4% of patients whose HBV-DNA had reached the therapeutic threshold, mother-to-child transmission hardly occurs. Liu’s study (14) shows that there were still cases of mother-to-child transmission among HBV-infected women whose HBV-DNA <5 log10IU/mL. Boucheron’s study (17) also proved that HBeAg is a good predictor of neonatal infection. If all HBeAg-positive mother were treated with antiviral treatment, the newborn’s infection will not occur. However, in our results, nearly 20% of HBeAg-positive pregnant women have HBV-DNA levels below 5.3 log10IU/mL. If the children of these patients undergo standardized immunization, the risk of MTCT is very low. They do not need to take antiviral drugs during pregnancy to avoid newborn infections. Many studies had proved that the safety of antiviral drugs used in pregnancy treatment is reliable (18,19). Whether HBeAg is suitable as a surrogate indicator for HBV-DNA detection needs to be comprehensively evaluated in terms of economic benefits. Peripartum antiviral prophylaxis is a short-term therapy, and if generic drugs are used, the price will be lower (1). From the perspective of WHO’s goal of eliminating hepatitis B by 2030, in countries and regions where it is difficult to fully implement HBV-DNA testing for pregnant women with hepatitis B, HBeAg is a satisfactory treatment indicator.
Among the studies we included, only one study (20) reported the mother-to-child transmission rate recorded by HBV-DNA classification based on the mother’s HBeAg status, more research is needed to explore whether there is a difference in the infection risk of children born to mothers with different HBeAg statuses at the same HBV-DNA level. To achieve the WHO’s goal of eliminating hepatitis B by 2030, further research is required to evaluate promising makers such as the HBsAg quantitative test, hepatitis B core-related Antigen is also worthy of consideration (21,22).
This study has some limitations. In some countries, there are few studies on the implementation rate of HBV DNA testing, and there are even no relevant studies in many regions. Moreover, the implementation rate of HBV DNA is affected by the time of research and local policies. To reflect the current popularity of HBV DNA testing in various regions, these studies are far from enough. But the researches we included can still reflect to a certain extent the popularity of HBV DNA testing among pregnant women with hepatitis B at a certain time in the local area. With reference to the time of studies, we can even explore the effects of some policies before and after implementation. The authors believe that these data also have merits. Our research did not search the literature according to the prevalence of HBeAg and the probability of MTCT of hepatitis B. Therefore, some data we summarized from existing articles cannot replace all comprehensive results of literatures in this area. But considering that in the articles we included, the number of documents reporting the positive rate of HBeAg and the rate of MTCT in pregnant women with hepatitis B is sufficient, and the subjects observed in these studies have a considerable scale, these data are also considered to be reliable. We also could not control the bias caused by the difference in detection time of HBV DNA during pregnancy because most of the included studies did not provide a specific time, but studies have shown that if antiviral treatment is not given, the level of HBV DNA during pregnancy is stable (23), therefore, the impact of detection time on the experimental results is negligible.
Conclusions
The implementation rate of HBV DNA testing varies from region to region. Limited studies show that HBV DNA testing does not cover all pregnant women with hepatitis B. When HBV-DNA testing is not available, it is worth considering to use HBeAg positivity as an antiviral therapeutic indicator among HBV-infected pregnant women for preventing MTCT.
Acknowledgments
We are thankful to researcher who generous share their study and data which was included in this study.
Funding: This study was supported by the Mega-Project of National Science and Technology for the 13th and 12th Five-Year Plan of China (grant number: 2018ZX10715-014-002 and 2014ZX10004008) and the National Natural Science Foundation of China (grant number: 81672005, U1611264, 81001271, 81721091).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1560
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1560). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. Global hepatitis report, 2017. Geneva: World Health Organization. 2017.
- WHO. Global health sector strategy on viral hepatitis 2016–2021. Geneva: World Health Organization. 2016.
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985;151:599-603. [Crossref] [PubMed]
- Childs L, Roesel S, Tohme RA. Status and progress of hepatitis B control through vaccination in the South-East Asia Region, 1992-2015. Vaccine 2018;36:6-14. [Crossref] [PubMed]
- Shimakawa Y, Lemoine M, Njai HF, et al. Natural history of chronic HBV infection in West Africa: a longitudinal population-based study from The Gambia. Gut 2016;65:2007-16. [Crossref] [PubMed]
- . Hepatitis B vaccines. Wkly Epidemiol Rec 2009;84:405-19. [PubMed]
- Park JS, Pan CQ. Viral factors for HBV mother-to-child transmission. Hepatol Int 2017;11:476-80. [Crossref] [PubMed]
- Chinese Society of Infectious Diseases CMA, Chinese Society of Hepatology CMA. The guidelines of prevention and treatment for chronic hepatitis B (2019 version). Chinese Journal of Hepatology 2019;27:938-61. [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-98. [Crossref]
- WHO. Prevention of mother-to-child transmission of hepatitis B virus: guidelines on antiviral prophylaxis in pregnancy. Geneva: World Health Organization. 2020.
- Kim H, Shin S, Oh EJ, et al. Comparison of the AdvanSure HBV real-time PCR test with three other HBV DNA quantification assays. Ann Clin Lab Sci 2013;43:230-7. [PubMed]
- Keshvari M, Alavian SM, Sharafi H. Comparison of Serum Hepatitis B Virus DNA and HBsAg Levels Between HBeAg-Negative and HBeAg-Positive Chronic Hepatitis B Patients. Jundishapur J Microbiol 2015;8:e21444 [Crossref] [PubMed]
- WHO. Guidelines on hepatitis B and C testing. Geneva: World Health Organization. 2017.
- Liu J, Yao N, Chen T, et al. Prevalence of mother-to-child transmission of hepatitis B virus: A systematic review and meta-analysis. J Hepatol 2019;70:e123-4. [Crossref]
- Latthaphasavang V, Vanhems P, Ngo-Giang-Huong N, et al. Perinatal hepatitis B virus transmission in Lao PDR: A prospective cohort study. PLoS One 2019;14:e0215011 [Crossref] [PubMed]
- Nayagam S, Shimakawa Y, Lemoine M. Mother-to-child transmission of hepatitis B: What more needs to be done to eliminate it around the world? J Viral Hepat 2020;27:342-9. [Crossref] [PubMed]
- Boucheron P, Lu Y, Yoshida K, et al. Accuracy of HBeAg to identify pregnant women at risk of transmitting hepatitis B virus to their neonates: a systematic review and meta-analysis. Lancet Infect Dis 2021;21:85-96. [Crossref] [PubMed]
- Sheng Q, Ding Y, Li B, et al. Efficacy and safety of nucleos(t)ide analogues to prevent hepatitis B virus mother-to-child transmission in pregnant women with high viremia: real life practice from China. Int J Med Sci 2018;15:796-801. [Crossref] [PubMed]
- Funk AL, Lu Y, Yoshida K, et al. Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: a systematic review and meta-analysis. Lancet Infect Dis 2021;21:70-84. [Crossref] [PubMed]
- Sun KX, Li J, Zhu FC, et al. A predictive value of quantitative HBsAg for serum HBV DNA level among HBeAg-positive pregnant women. Vaccine 2012;30:5335-40. [Crossref] [PubMed]
- Yang XH, Shi XF. Significance of HBsAg quantification in guiding clinical treatment of chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi 2016;24:317-20. [PubMed]
- Yoshida K, Desbiolles A, Feldman SF, et al. Hepatitis B Core-Related Antigen to Indicate High Viral Load: Systematic Review and Meta-Analysis of 10,397 Individual Participants. Clin Gastroenterol Hepatol 2021;19:46-60.e8. [Crossref] [PubMed]
- Giles M, Visvanathan K, Lewin S, et al. Clinical and virological predictors of hepatic flares in pregnant women with chronic hepatitis B. Gut 2015;64:1810-5. [Crossref] [PubMed]



