
Toxic epidermal necrolysis with systemic lupus erythematosus: case report and review of the literature
Introduction
Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS) are potentially fatal mucocutaneous diseases characterized by extensive necrosis and exfoliation of the epidermis. TEN and SJS are most often caused by various kinds of drugs (1,2). SJS and TEN were first identified in 1922 (1), but at present, a widely recognized view is that SJS and TEN represent phases in the continuous progress of the same disease (1). The incidence of SJS and TEN, respectively, have been reported to be 1–7 and 1–2 cases per million people (3). The degree of total body surface area (TBSA) involved determines where on the SJS/TEN spectrum a patient lies (SJS: <10% TBSA; SJS-TEN overlap: 10–30% TBSA; TEN: >30% TBSA) (4). The Nationwide Inpatient Sample 2009–2012 (U.S.) statistics show that the mean adjusted mortality was 4.8% for SJS, 19.4% for SJS/TEN overlap, and 14.8% for TEN (5).
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease of unknown etiology that affects almost all organs. It is characterized by abnormal autoimmune antibodies and varied clinical manifestations. While SJS/TEN is rare, cases of SJS/TEN combined with SLE are even less common. Occasionally acute cutaneous manifestations of SLE and SJS ⁄TEN can be phenotypically similar, both causing extensive epidermal necrosis. Although no feature by itself is conclusive, a combination of recent SLE exacerbation, evident photodistribution, annular lesions, and absent or only mild focal erosive mucosal involvement may favour SLE over SJS ⁄TEN clinically. In this paper, we present a recent case of a 32-year-old female TEN-with-SLE patient, and review related studies to expand clinicians’ approach to diagnosis and treatment. We present the following case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-341/rc).
Case presentation
A 32-year-old female was admitted to the emergency department with increased eye secretion, sore throat for 10 days, submandibular ulceration, and 9 days of mental disorder exhibited by the inability to communicate. At admission, the patient had mild facial edema accompanied by a red rash all over the body, and slightly ruptured skin in the mandibular region. The skin damage had progressed quickly: the wound surface had expanded rapidly to the whole body, accounting for 70–80% of the body surface. Within 3 days, numerous blisters had appeared on the patient’s skin, and a large area of epidermal mucosa was exfoliated. She reported that 14 years ago she had been diagnosed with SLE, and had been taking 5 mg prednisone acetate tablets orally every day since, for the last 14 years. The patient additionally self-administered the health product astaxanthin for the last two months. She denied any history of food or drug allergies, or adverse familial genetic history, though her parents’ marriage was consanguineous.
On physical examination, the patient’s body temperature was 37.5 ℃, pulse 126 beats per minute, blood pressure 121/80 mmHg, and SpO2 100%. A laboratory examination showed the hemoglobin level at 10.3 g/dL (reference range, 11.5–15.0 g/dL), leukocyte count 8,450/mm3 (reference range, 3,500–9,500/mm3; 84.4% neutrophils, 0% eosinophils), and platelet count (Plt) 126,000/mm3 (reference range, 125,000–350,000/mm3). The erythrocyte sedimentation rate was 28 (reference range, 0–20) mm per hour and C-reactive protein (CRP) was 115 mg/L (reference range, ≤8 mg/L). Autoimmune markers were positive for anti-nuclear antibody (ANA) 1:640, speckled, anti-Smith, and anti-Ro/La. Anti-double-stranded DNA (anti-dsDNA) and anti-ribonucleoprotein (anti-RNP) were negative. C3 was 62.6 mg/dL (reference range, 85–200 mg/dL) and C4 was 24.2 mg/dL (reference range, 12–40 mg/dL). Both the patient’s blood cultures and blister culture showed Acinetobacter baumannii.
The SCORTEN (SCORE of Toxic Epidermal Necrosis) was 3 points: tachycardia above 120 beats per min, percentage of epidermal detachment above 10% at admission, serum urea level above 10 mmol per liter. A diagnosis of TEN was made, and the patient was given supportive care and adjuvant therapy immediately. It is worth mentioning that we used compound polymyxin B ointment for skin treatment, as it contains lidocaine, which can significantly reduce the pain while also being an antibacterial. We applied it to the wound to reduce fluid loss and to provide a moist wound environment to optimize re-epithelialization. Pain and consciousness disorder caused dys-expectoration, which made it impossible for the patient to discharge sputum. She secreted so much that she soon developed respiratory failure. Tracheal intubation was used to keep the airway open to assist breathing, and enteral nutrition was given. Adjuvant therapy included intravenous (IV) methylprednisolone (60 mg/day) for nine days, as well as IV immunoglobulin (IVIG) 400 mg/kg/day for twelve days and plasma exchange treatment. Imipenem and cilastatin sodium, tigecycline, and sulbactam were used in combination for infection. After three weeks, the patient’s cutaneous lesions improved significantly (Figures 1,2) and she was able to coordinate many movements. The patient was transferred to the rheumatology ward.
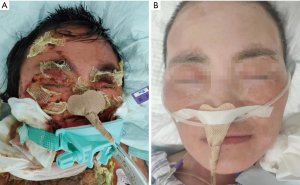
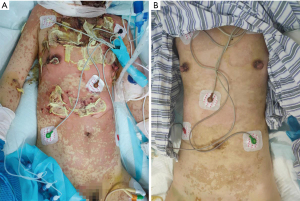
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient. Figures 1,2 are shown with the consent of the patient and her family. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
To provide context to this paper’s presentation of a recent case of TEN/SJS with SLE, we have reviewed relevant case studies, accessed via PubMed databases. The keywords for the search were Stevens-Johnson syndrome/toxic epidermal necrolysis AND systemic lupus erythematosus. The search covers all case studies published in English from 1988 to 2019. Key information is presented in Table 1.
Table 1
| Case | Reference | Demo-graphics | ANA titer | Blood cultures | Treatment | Drugs/period administered | Results | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Systemic steroids | IVIG | Immune-suppressor | Plasma-pheresis | |||||||
| 1 | K Aiempanakit | 60 y/F | ≥1:1,280 homogeneous and nucleolar | Positive (Acinetobacter baumannii) | √ | Aspirin, nifedipine, colchicine, naproxen/2 w | Died | |||
| 2 | Samimi SS | 9 y/F | >1:640 | √ | √ | √ | √ | Azithromycin/2 w | Improved | |
| 3 | Mersedeh Moshfeghi | 31 y/F | √ | Ciprofloxacin/6 d | Improved | |||||
| 4 | Kim M.Murray | 32 y/F | 1:640 speckled | √ | Cephalexin/6 d | Improved | ||||
| 5 | Savill JS | 60 y/F | >1:320 diffuse | √ | √ | Iopamidol/8 h | Died | |||
| 6 | Hemmige V | 49 y/F | 1:2,560 speckled | Positive | √ | Herbal medications | Improved | |||
| 7 | Lateef A | 67 y/F | √ | √ | Cyclosporine/3 w | Improved | ||||
| 8 | S Paradela | 72 y/F | 1:320 nucleolar | Positive (Staphylococcus aureus) | √ | Gabapentin/3 w | Improved | |||
| 9 | Goldberg D | 62 y/F | √ | √ | Rofecoxib/3 w | Improved | ||||
| 10 | Mandelcorn R | 42 y/F | 1:2,560 homogeneous | √ | √ | Improved | ||||
| 11 | Mandelcorn R | 76 y/F | 1:320 speckled | √ | √ | √ | √ | Improved | ||
| 12 | Baker MG | 24 y/F | Positive | Positive (Staphylococcus aureus) | √ | Improved | ||||
| 13 | Rubio-Gonzalez B | 43 y/F | Positive | √ | √ | Improved | ||||
| 14 | Rubio-Gonzalez B | 29 y/F | Positive | √ | √ | Improved | ||||
| 15 | Yildirim Cetin G | 52 y/F | Positive | √ | √ | √ | Improved | |||
| 16 | Bhasin A | 28 y/F | 2+ | Negative | Mirtazepine/15 d | Improved | ||||
| 17 | Boontaveeyuwat E | 35 y/F | 1:2,560 homogeneous and peripheral | √ | Improved | |||||
| 18 | Boontaveeyuwat E | 60 y/F | 1:2,560 homogeneous and peripheral | √ | √ | Amlodipine, omeprazole, folic acid, feso4/2 m | Improved | |||
| 19 | Konda S | 21 y/F | 1:640 | √ | √ | Improved | ||||
| 20 | Cavalcante EG | 18 y/F | 1:640 dense fine speckled | Positive (MASA) | √ | Linezolid, Trimethoprim-sulfamethoxazole/4 d | Died | |||
| 21 | Lee HY | 12 y/F | 1:800 speckled | √ | √ | Improved | ||||
| 22 | Lee HY | 47 y/F | 1:400 homogeneous 1:800 speckled | √ | √ | Improved | ||||
| 23 | Lee HY | 31 y/F | 1:800 speckled | √ | √ | Cloxacillin, ceftriaxone/2 d | Improved | |||
| 24 | Tenea D | 39 y/F | Negative | √ | Carbamazepine/11 d | Improved | ||||
| 25 | Marija S | 14 y/F | 1:640 | Positive (Pseudomonas aeruginosa) | √ | √ | Ampicillin, amikacin, metronidazole/2 d | Died | ||
| 26 | Yu J | 15 y/F | Positive | √ | √ | √ | Hydroxychloroquine/4 d | Improved | ||
| 27 | Yu J | 11 y/F | Positive | √ | √ | √ | Prednisone, hydroxychloroquine/4 m, mycophenolate mofetil/1 m | Improved | ||
| 28 | Yu J | 18 y/M | √ | √ | Clindamycin/2 w | Improved | ||||
| 29 | Jang HW | 16 y/F | 1:640 homogeneous | √ | √ | Improved | ||||
| 30 | Vazquez-Sanabria IL | 38 y/F | Negative | √ | Azathioprine/2 w | |||||
ANA, antinuclear antibodies; IVIG, IV immunoglobulin; y, years; F, female; M, male; h, hours; d, days; m, months; w, weeks.
A total of 30 cases were collected from the literature, the patient sample having a male-female ratio of 1:29 and an average age of 37. As is evident in Table 1, most of the patients had a suspected history of medication before onset, and their treatment mainly consisted of glucocorticoids, IVIGs, and immune-suppressors. Four patients died, but most had good prognosis and were cured at discharge.
Although SJS/TEN is a severe skin mucous membrane response mainly triggered by drugs, some cases cannot be attributed to a particular drug (3). Other known risk factors for SJS/TEN include pneumonia infection, HIV infection, genetic factors, tumors, and underlying immune diseases (6-10). Parperis et al. reviewed the January 2013 to December 2018 medical records of 30 patients diagnosed with autoimmune rheumatic diseases at an Arizona burn center. TEN/SLE was triggered in the two patients with systemic lupus erythematosus. The study showed that patients with autoimmune rheumatic diseases were more likely to develop TEN/SLE under the influence of a drug (11). In over one-third of SJS/TEN cases, however, no risk factor can be identified (12).
While the pathogenesis of SJS/TEN remains unclear, some recent studies suggest that exosomes isolated from the plasma of SJS/TEN patients with SJS/TEN were 30 to 200 nm in diameter, and expressed CD9, CD63, CD81, and TSG101 and TSG101 exosome marker proteins. MiR-375-3p has been shown to be markedly up-regulated in patients with SJS/TEN, and to be correlated with clinical severity (13). In relation to autoimmune diseases, SJS/TEN patients have T cell dysfunction, in which CD8+ cytotoxic T lymphocytes are the main effector cells, manifested as an elevated level of tumor necrosis factor (TNF) (14). Another pathological feature is apoptotic keratin leading to separation of epidermis and dermis. The changes in the epidermis are related to vacuolar degeneration of basal cells. In the dermis of SJS/TEN patients, there is usually only a small infiltration of eosinophils, mainly perivascular lymphocytes and histological cells (15). Prodromal symptoms of SJS/TEN are fever and influenza-like symptoms 1–3 days before the occurrence of skin mucosal lesions (2). Patients with TEN are prone to multiple organ complications, in addition to skin lesions (16).
As no guidelines exist for primary treatment, identification and discontinuation of the offending medication(s) should be a priority. Attention should be paid to adjuvant symptomatic support therapy, pain management, and prevention of infection. Adjuvant therapy includes systemic corticosteroids, immunosuppressants, tumor necrosis factor inhibitors, and plasmapheresis, as well as intravenous immunoglobulin (IVIG), which can inhibit the apoptosis of keratinocytes. It is known that the death receptors Fas and ligand FasL can mediate keratinocyte apoptosis; specifically, antagonistic anti-Fas antibody or IVIG containing natural anti-Fas antibody can inhibit keratinocyte apoptosis in vitro (17). However, a recent systematic review and meta-analysis suggests glucocorticosteroids and cyclosporine were the most promising systemic immunomodulating therapies for SJS/TEN, while no beneficial findings were observed for other therapies, including IVIG (18). Psychological factors related to post-traumatic stress disorder probably play a key role in persistent pain, which has been found to be mainly located at the eyes (55%), mouth, and lower limbs (38–41%), with moderate intensity on average (19). Ophthalmic management, according to the “UK Guidelines for the Management of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis in Adults 2016”, requires the minimization of destructive ocular surface and lid margin inflammation, the management and prevention of conjunctival adhesions, infection prophylaxis, and the prompt identification and management of the blinding complications of corneal exposure, ulceration, and infection (15).
The mortality of SJS/TEN is predicted by the extent and severity of symptoms at presentation, as assessed using SCORTEN. SCORTEN evaluates the severity of illness and risk of death based on seven clinical and biological parameters (20) that clinicians should assess within 24 hours of admission: age above 40 y, presence of malignancy, tachycardia above 120 per min, percentage of epidermal detachment above 10% at admission, serum urea level above 10 mmol per liter, serum glucose level above 14 mmol per liter, and bicarbonate level below 20 mmol per liter. The most common causes of death in the hospital are sepsis, acute respiratory distress syndrome, and multiple organ failure (2,5,21). The need for a patient’s mechanical ventilation is also associated with higher mortality (4).
The patient described in this case report had a history of systemic lupus erythematosus and suspected astaxanthin medication history. In susceptible individuals, the carotenoids in astaxanthin may release toxic retinoic acid compounds into the circulation, leading to endogenous hypervitamin A, thereby mediating cytotoxicity, which is considered a potential pathological feature of SJS/TEN. The early indicators of this patient’s disease were eye and submandibular bursts. The ulceration of the chest skin was serious, while ulceration of the limbs was light and superficial. She was diagnosed with TEN combined with SLE after presentation of the above symptoms, six months after using astaxanthin. After treatment with glucocorticoid, immunoglobulin, plasma exchange, and other treatments recommended by the “UK Guidelines for the Management of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis in Adults 2016” (15), the patient recovered quickly with supportive care and adjunctive therapies. She had improved significantly 3 weeks later and was doing well at her three-month follow-up.
We had great success in caring for the skin with extensive mucosal exfoliation, while reducing pain and fighting infection. This case can guide future clinical practice. However, through our search of the literature, it is clear that the mechanism, immunity, and treatment of SJS/TEN are still not fully understood and need further study.
Acknowledgments
We would like to thank Bai Yi for her help in polishing our paper. We also thank the medical staff for their hard work on this patient.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-341/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-341/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-341/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee (s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Murray KM, Camp MS. Cephalexin-induced Stevens-Johnson syndrome. Ann Pharmacother 1992;26:1230-3. [Crossref] [PubMed]
- Oakley AM, Krishnamurthy K. Stevens Johnson Syndrome. StatPearls. Treasure Island (FL), 2021.
- Stern RS, Divito SJ. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Associations, Outcomes, and Pathobiology-Thirty Years of Progress but Still Much to Be Done. J Invest Dermatol 2017;137:1004-8. [Crossref] [PubMed]
- Shanbhag SS, Chodosh J, Fathy C, et al. Multidisciplinary care in Stevens-Johnson syndrome. Ther Adv Chronic Dis 2020;11:2040622319894469. [Crossref] [PubMed]
- Hsu DY, Brieva J, Silverberg NB, et al. Morbidity and Mortality of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in United States Adults. J Invest Dermatol 2016;136:1387-97. [Crossref] [PubMed]
- Ferrandiz-Pulido C, Garcia-Patos V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch Dis Child 2013;98:998-1003. [Crossref] [PubMed]
- Roujeau JC. Stevens-Johnson syndrome and toxic epidermal necrolysis are severity variants of the same disease which differs from erythema multiforme. J Dermatol 1997;24:726-9. [Crossref] [PubMed]
- Chowdhury AD, Oda M, Markus AF, et al. Herbal medicine induced Stevens-Johnson syndrome: a case report. Int J Paediatr Dent 2004;14:204-7. [Crossref] [PubMed]
- Ball R, Ball LK, Wise RP, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis after vaccination: reports to the vaccine adverse event reporting system. Pediatr Infect Dis J 2001;20:219-23. [Crossref] [PubMed]
- Oda T, Sawada Y, Okada E, et al. Stevens-Johnson Syndrome After Influenza Vaccine Injection. J Investig Allergol Clin Immunol 2017;27:274-5. [Crossref] [PubMed]
- Parperis K, Bhattarai B, Hadi M, et al. Burn center admissions of patients with autoimmune rheumatic diseases: clinical characteristics and outcomes. Rheumatol Int 2020;40:1649-56. [Crossref] [PubMed]
- Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther 2010;88:60-8. [Crossref] [PubMed]
- Zhang C, Zhu Z, Gao J, et al. Plasma exosomal miR-375-3p regulates mitochondria-dependent keratinocyte apoptosis by targeting XIAP in severe drug-induced skin reactions. Sci Transl Med 2020;12:eaaw6142. [Crossref] [PubMed]
- Lateef A, Tan KB, Lau TC. Acute generalized exanthematous pustulosis and toxic epidermal necrolysis induced by hydroxychloroquine. Clin Rheumatol 2009;28:1449-52. [Crossref] [PubMed]
- Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. J Plast Reconstr Aesthet Surg 2016;69:e119-53. [Crossref] [PubMed]
- Yildirim Cetin G, Sayar H, Ozkan F, et al. A case of toxic epidermal necrolysis-like skin lesions with systemic lupus erythematosus and review of the literature. Lupus 2013;22:839-46. [Crossref] [PubMed]
- Sato S, Kanbe T, Tamaki Z, et al. Clinical features of Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr Int 2018;60:697-702. [Crossref] [PubMed]
- Zimmermann S, Sekula P, Venhoff M, et al. Systemic Immunomodulating Therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis. JAMA Dermatol 2017;153:514-22. [Crossref] [PubMed]
- Lefaucheur JP, Valeyrie-Allanore L, Ng Wing Tin S, et al. Chronic pain: a long-term sequela of epidermal necrolysis (Stevens-Johnson syndrome/toxic epidermal necrolysis) - prevalence, clinical characteristics and risk factors. J Eur Acad Dermatol Venereol 2021;35:188-94. [Crossref] [PubMed]
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol 2000;115:149-53. [Crossref] [PubMed]
- Sekula P, Dunant A, Mockenhaupt M, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol 2013;133:1197-204. [Crossref] [PubMed]


