
Predictive value of red blood cell distribution width level on histological response and prognosis in colorectal liver metastases
Introduction
Colorectal cancer ranks third in terms of incidence and second in terms of mortality among all cancers worldwide (1). More than 50% of patients diagnosed with colorectal cancer are affected by metastatic disease, and the five-year survival rate is approximately 13% (1,2). The liver is the first and most common target organ for metastasis, which is a significant cause of death (3). Liver resection remains the most curative option for colorectal liver metastases (CRLMs), with a 5-year survival rate of approximately 43.3% (4). Nevertheless, the recurrence rate is greater than 70% after surgery (4), and over half of patients develop recurrence within 2 years (5). In addition, major postoperative complications, which have substantial adverse effects on the quality of postoperative life of CRLM patients, occur at a rate of approximately 17.0–19.9% (4,6). Therefore, it is necessary to effectively predict the outcomes of patients with CRLM in advance and to adjust prevention or treatment strategies in this specific clinical scenario.
Neoadjuvant chemotherapy (NAC) is widely administered in CRLM patients with a high risk of recurrence to eliminate micrometastases and induce tumour shrinkage (7). Accumulating evidence unequivocally demonstrates that NAC can significantly prolong the survival of CRLM patients who undergo liver resection (7-9). Pathological response is an important factor in the evaluation of chemotherapy efficacy and the prediction of outcomes in patients treated with NAC followed by liver resection (10,11). A favorable pathological response, which has been recognized as a predictor of improved postoperative survival, was observed in 45–57% of these patients (12,13). Therefore, it is essential to identify more reliable indicators to predict the pathological outcomes of CRLM patients receiving NAC, with the intention of selecting more suitable patients for NAC and liver resection.
Many studies have investigated the predictive value of pre-operative testing markers in CRLM patients. Studies have indicated that inflammation plays an indispensable role in the development, invasion, and metastasis of colorectal cancer (14,15). Pre-operative inflammatory biomarkers, the neutrophil-to-lymphocyte ratio, the platelet-to-lymphocyte ratio, and the C-reactive protein-to-lymphocyte ratio, among others, have been shown to present a strong correlation with the prognosis of patients with CRLM (16,17). In addition, the elevated pre-operative GGT and D-dimer were associated with post-operative major complications and worse survival (4). Red blood cell distribution width (RDW) is a direct reflection of the red blood cell size distribution. An increased RDW mirrors a disorder of red blood cell homeostasis ascribed to various potential abnormal metabolic processes (18). RDW is not only a diagnostic parameter for distinguishing different types of anemia but also an important inflammatory biomarker in various diseases (19,20). Some studies have revealed that RDW was a potential prognostic factor for diverse hematological malignancies (21) and solid tumours, including liver (22), esophageal (23) and colorectal tumours (24). However, previous studies focused only on the predictive value of the preoperative RDW level on prognosis and did not investigate the effect of the pre-neoadjuvant chemotherapy (pre-NAC) RDW level or the fluctuation in the RDW level during chemotherapy. In addition, there is no study focusing on the predictive value of RDW level in CRLM patients. Pre-NAC testing markers and the fluctuation in testing markers during NAC have a potentially predictive value for pathological response and survival in CRLM patients (6,11,12). Consequently, we sought to comprehensively determine the value of RDW in CRLM patients by evaluating the predictive value of the pre-neoadjuvant chemotherapy (pre-NAC) RDW level, the preoperative RDW level and the fluctuation between the pre-NAC RDW level and the preoperative RDW level. The aim was to determine whether the RDW level could be used to predict a pathological response as well as the outcomes of CRLM patients. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-934).
Methods
Patients
This is a retrospective study based on the CRLM database. This study was approved by the Institutional Review Board of the Cancer Institute & Hospital, Chinese Academy of Medical Sciences (ID: NCC2019C-016). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent has been obtained from patients. Clinicopathologic data, treatments and outcomes of CRLM patients treated with NAC followed by liver resection at our hospital between January 2010 and December 2017 were reviewed. The inclusion criteria were as follows: (I) pathologically proven colorectal adenocarcinoma liver metastases; (II) resection of primary colorectal cancer; and (III) administration of NAC followed by liver resection for curative intent. The exclusion criteria were as follows: (I) diagnosis of other malignancies; (II) lack of follow-up and clinical data; and (III) presentation with pre-treatment comorbidities known to be associated with a change in the RDW level (e.g., anaemia, cardiovascular diseases). One hundred fifty patients were included in our study.
RDW level
The RDW level was presented as the RDW-SD level and the RDW-CV level. Pre-NAC and preoperative serum RDW-CV levels (normal range, 11.6–14.6%) and RDW-SD levels (normal range, 37.0–51.0 fl) were measured simultaneously within 1 week prior to NAC or surgery. According to the change between the pre-NAC RDW level and the preoperative RDW level, the RDW change was scored as 0 (both pre-NAC and preoperative RDW level < cut-off), 1 (pre-NAC RDW level < cut-off and preoperative RDW level ≥ cut-off; pre-NAC RDW level ≥ cut-off and preoperative RDW level < cut-off) or 2 (both pre-NAC and preoperative RDW level ≥ cut-off).
Treatment
The NAC regimens primarily consisted of a combination of 5-fluorouracil/capecitabine and oxaliplatin/irinotecan with or without targeted agents such as bevacizumab or cetuximab. It was recommended that patients with initially unresectable CRLM and those with multiple high-risk recurrence factors receive NAC. The clinical response to NAC was evaluated by CT/MRI scans and the Response Evaluation Criteria in Solid Tumours (RECIST 1.1) (25). The clinical responses were classified as complete response, partial response, stable disease or progressive disease. A favorable clinical response was defined as either complete response or partial response. The pathological response to NAC was evaluated according to the tumour regression grade (TRG) after surgery (10). TRG included grades 1–5, and TRG1–3 was defined as a favorable pathological response. Within 4–6 weeks after NAC completion, the patients underwent liver resection. Liver resection was defined as major or minor liver resection. Resections of fewer than two liver segments were regarded as minor liver resections.
Follow-up and outcomes
Patients were asked to undergo regular clinical examinations: one month after surgery was the first follow-up date; then, examinations were recommended every 3 months for the first 2 years, every 6 months for the next 3 years, and every year thereafter. CEA levels and imaging examinations were used to detect any progression or recurrence after surgery. The primary outcomes were progression-free survival (PFS) and overall survival (OS). PFS was defined as the date of resection to the date of progression or the last follow-up. OS was defined as the date of resection to the date of death or the last follow-up. The secondary outcome was postoperative complications. Postoperative complications were graded according to the Clavien-Dindo system (I to V) (26) and included minor complications and major complications. Postoperative complications of grade III to V were defined as major complications.
Statistical analysis
Statistical analysis was performed using SPSS version 22 software (Armonk NY, USA) and R software (http://www.r-project.org). Continuous variables were tested for normal distribution, conforming to the normal distribution using t test, and not conforming to the normal distribution using Mann-Whitney U test. The chi-square test or Fisher’s exact test was used to analyse categorical variables. The categorical variables included age, sex, BMI, comorbidity, preoperative testing markers, T stage, pathological response, etc. (Table 1), the comparison was made in these groups. The optimal cutoff values of RDW-SD and RDW-CV for survival were determined by X-tile analysis. X-tile analysis divided RDW level into two populations: low and large. The associations of all possible divisions with survival can be calculated by the log-rank test. The optimal division of the data was selected by the highest X2 value. The PFS and OS curves were constructed using the Kaplan-Meier method, and the differences between two groups were calculated by the log-rank test. All factors with P<0.10 in the univariate analysis were included in the multivariate analysis. Multivariable logistic regression analysis was performed to determine the relationships between tumour characteristics and pathological response and the major postoperative complications. A multivariate Cox proportional hazards model was used to evaluate the prognostic factors associated with survival. A forward likelihood ratio was implemented in the multivariate analysis. A value of two-sided P<0.05 indicated a statistically significant difference.
Table 1
| Factor | All patients, n=150 | RDW-CV change =0,1, n=112 (74.7%) | RDW-CV change =2, n=38 (25.3%) | P value |
|---|---|---|---|---|
| Age ≥60 years, n (%) | 54 (36.0) | 41 (36.6) | 13 (34.2) | 0.790 |
| Male, n (%) | 103 (68.7) | 82 (73.2) | 21 (55.3) | 0.039 |
| BMI ≥24 kg/m2, n (%) | 82 (54.7) | 61 (54.5) | 21 (55.3) | 0.932 |
| Comorbidity, n (%) | 69 (46.0) | 58 (51.8) | 11 (28.9) | 0.015 |
| Preoperative CEA ≥10 ng/mL, n (%) | 64 (42.7) | 49 (43.8) | 15 (39.5) | 0.645 |
| Pre-NAC RDW-SD ≥42.20 fl, n (%) | 55 (36.7) | 28 (25.0) | 27 (71.1) | <0.001 |
| Preoperative RDW-SD ≥42.20 fl, n (%) | 136 (90.7) | 98 (87.5) | 38 (100.0) | 0.022 |
| RDW-SD change =2, n (%) | 50 (33.3) | 23 (20.5) | 27 (71.1) | <0.001 |
| Pre-NAC RDW-CV ≥13.5%, n (%) | 46 (30.7) | 8 (7.1) | 38 (100.0) | <0.001 |
| Preoperative RDW-CV ≥13.5%, n (%) | 108 (72.0) | 70 (62.5) | 38 (100.0) | <0.001 |
| Right hemicolon, n (%) | 18 (12.0) | 11 (9.8) | 7 (18.4) | 0.159 |
| Poor differentiation, n (%) | 44 (29.3) | 35 (31.3) | 9 (23.7) | 0.376 |
| T3–T4 stage, n (%) | 126 (84.0) | 91 (81.3) | 35 (92.1) | 0.115 |
| Primary lymph node metastasis, n (%) | 113 (75.3) | 89 (79.5) | 24 (63.2) | 0.044 |
| Synchronous metastasis, n (%) | 135 (90.0) | 100 (89.3) | 35 (92.1) | 0.617 |
| Diameter of metastases ≥3 cm, n (%) | 67 (44.7) | 47 (42.0) | 20 (52.6) | 0.253 |
| Multiple metastases, n (%) | 105 (70.0) | 79 (70.5) | 26 (68.4) | 0.806 |
| Bilobar liver distribution, n (%) | 73 (48.7) | 56 (50.0) | 17 (44.7) | 0.575 |
| Extrahepatic metastases, n (%) | 17 (11.3) | 12 (10.7) | 5 (13.2) | 0.681 |
| Heterochronous resection, n (%) | 39 (26.0) | 28 (25.0) | 11 (28.9) | 0.632 |
| R0 resection, n (%) | 99 (66.0) | 73 (65.2) | 26 (68.4) | 0.715 |
| Major liver resection, n (%) | 81 (54.0) | 60 (53.6) | 21 (55.3) | 0.857 |
| Concomitant RFA, n (%) | 29 (19.3) | 23 (20.5) | 6 (15.8) | 0.522 |
| Operation time ≥339 min, n (%) | 75 (50.0) | 56 (50.0) | 19 (50.0) | 1.000 |
| Blood loss ≥250 mL, n (%) | 75 (50) | 52 (46.4) | 23 (60.5) | 0.133 |
| Intraoperative blood transfusion, n (%) | 19 (17.0) | 13 (34.2) | 32 (21.3) | 0.025 |
| Major postoperative complications, n (%) | 32 (21.3) | 20 (17.9) | 12 (31.6) | 0.074 |
| Postoperative complications, n (%) | 83 (55.3) | 58 (51.8) | 25 (65.8) | 0.134 |
| Oxaliplatin-based regimen, n (%) | 101 (67.3) | 74 (66.1) | 27 (71.1) | 0.572 |
| NAC cycles ≥4, n (%) | 110 (73.3) | 85 (75.9) | 25 (65.8) | 0.224 |
| Targeted therapy, n (%) | 37 (33.0) | 13 (34.2) | 50 (33.3) | 0.894 |
| Postoperative chemotherapy, n (%) | 85 (56.7) | 56 (50.0) | 29 (76.3) | 0.005 |
| Favourable clinical response, n (%) | 56 (50.0) | 19 (50.0) | 75 (50.0) | 1.000 |
| Favourable pathological response, n (%) | 64 (42.7) | 44 (39.3) | 20 (52.6) | 0.151 |
CEA, carcinoembryonic antigen; NAC, pre-neoadjuvant chemotherapy; RDW, red blood cell distribution width.
Results
Patient and tumour characteristics
Of the 150 patients included in this study, 103 were men (68.3%) and 47 were women (31.7%), with a mean age of 55.7±10.1 years. A BMI ≥24 kg/m2 was observed in 54.7% of the patients (82/150), and 46.0% of the patients (69/150) had comorbidities. Most patients (90.0%) had synchronous liver metastases. In all, 70.0% of the patients (105/150) had more than one liver metastasis, with a median liver metastasis number of 3.0 (IQR 1.0–4.0) and a median liver metastasis diameter of 2.5 (IQR 1.7–4.0) cm. A bilobar liver metastasis distribution was observed in 48.7% of the patients (73/150). Poor differentiation was observed in 29.3% of the patients (44/150). A primary tumour stage of T3-T4 was observed in 126 patients (84.0%). Major liver resection was performed in 54.0% of the patients. The median operation time, median blood loss during surgery and percentage of patients who received an intraoperative blood transfusion were 339.0 (IQR 258.8–406.0) min, 250.0 (IQR 100–500) mL and 17.0%, respectively. One hundred and one patients (67.3%) received an oxaliplatin-based regimen, and 37 patients (33.0%) received targeted therapy. The median number of NAC cycles was 4, and 110 patients (73.3%) received more than 4 NAC cycles. Eighty-five patients (56.7%) received postoperative chemotherapy. A favorable pathological response was observed in 42.7% of the patients (64/150) (Table 1).
Characteristics of the pre-NAC RDW level and the preoperative RDW level
The mean pre-NAC RDW-CV level and the mean preoperative RDW-CV level were 13.9±3.5 and 15.1±2.4, respectively (P<0.001). The mean pre-NAC RDW-SD level and the preoperative RDW-SD level were 41.9±6.3 and 50.9±7.8, respectively (P<0.001). The optimal cut-offs of the pre-NAC RDW-CV level and the pre-NAC RDW-SD level for survival were 13.5% and 42.2 fl, respectively. A pre-NAC RDW-CV ≥13.5% and a preoperative RDW-CV ≥13.5% were observed in 46 patients (30.7%) and 108 patients (72.0%), respectively. A pre-NAC RDW-SD ≥42.2 fl and a preoperative RDW-SD ≥42.2 fl were observed in 55 patients (36.7%) and 136 patients (90.7%), respectively.
According to the change between the pre-NAC RDW level and the preoperative RDW level, patients were then categorized into the following groups: RDW-CV change =0 (both a pre-NAC and preoperative RDW-CV <13.5%), 34 patients (22.7%); RDW-CV change =1 (a pre-NAC RDW-CV <13.5% and a preoperative RDW-CV ≥13.5%, a pre-NAC RDW-CV ≥13.5% and a preoperative RDW-CV <13.5%), 78 patients (52.0%); RDW-CV change =2, 38 patients (25.3%); RDW-SD change =0, 9 patients (6.0%); RDW-SD change =1, 91 patients (60.7%); and RDW-SD change =2, 50 patients (33.3%). An RDW-CV change =2 was significantly associated with female sex (P=0.039), non comorbidity (P=0.015), non primary lymph node metastasis (P=0.044) and a small number of postoperative chemotherapy cycles (P=0.005) (Table 1).
Predictive value of the pre-NAC RDW level and the preoperative RDW level on the histological response
The univariate analysis revealed that a pre-NAC RDW-SD ≥42.20 fl (P=0.873), a preoperative RDW-SD ≥42.20 fl (P=0.105), an RDW-SD change =2 (P=0.815), a pre-NAC RDW-CV ≥13.5% (P=0.625) and an RDW-CV change =2 (P=0.153) were not significantly associated with a pathological response. A preoperative RDW-CV ≥13.5% was significantly associated with a favorable pathological response (OR =2.716, 95% CI: 1.239–5.951, P=0.013). The univariate analysis also revealed that a primary tumour site in the right hemicolon (P=0.011), a preoperative CEA ≥10 ng/mL (P=0.036), targeted therapy (P<0.001) and clinical response (P=0.001) were correlated with a favorable histological response. The multivariate analysis showed that a preoperative RDW-CV ≥13.5% (OR =3.215, 95% CI: 1.299–7.958, P=0.012) significantly predicted a favorable pathological response, and a BMI ≥24 kg/m2 (OR =0.445, 95% CI: 0.208–0.953, P=0.037), primary tumour site in the right hemicolon (OR =4.859, 95% CI: 1.389–16.997, P=0.013), targeted therapy (OR =4.354, 95% CI: 1.944–9.751, P<0.001) and clinical response (OR =2.522, 95% CI: 1.188–5.353, P=0.016) were independent predictors of a histological response (Table 2).
Table 2
| Factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | ||
| Age ≥60 years | 0.989 | 0.995 (0.507–1.953) | – | – | |
| Male | 0.985 | 1.007 (0.501–2.023) | – | – | |
| BMI ≥24 kg/m2 | 0.099 | 0.577 (0.300–1.110) | 0.037 | 0.445 (0.208–0.953) | |
| Comorbidity | 0.142 | 0.612 (0.317–1.180) | – | – | |
| Preoperative CEA ≥10 ng/mL | 0.036 | 0.488 (0.250–0.956) | – | – | |
| Pre-NAC RDW-SD ≥42.20 fl | 0.873 | 0.947 (0.483–1.854) | – | – | |
| Preoperative RDW-SD ≥42.20 fl | 0.105 | 2.982 (0.796–11.170) | – | – | |
| RDW-SD change =2 | 0.815 | 1.085 (0.547–2.153) | – | – | |
| Pre-NAC RDW-CV ≥13.5% | 0.623 | 1.192 (0.592–2.398) | – | – | |
| Preoperative RDW-CV ≥13.5% | 0.013 | 2.716 (1.239–5.951) | 0.012 | 3.215 (1.299–7.958) | |
| RDW-CV change =2 | 0.153 | 1.717 (0.818–3.603) | – | – | |
| Right hemicolon | 0.011 | 4.129 (1.389–12.273) | 0.013 | 4.859 (1.389–16.997) | |
| Poor differentiation | 0.016 | 0.389 (0.181–0.837) | – | – | |
| T3–T4 stage | 0.217 | 0.575 (0.239–1.385) | – | – | |
| Primary lymph node metastasis | 0.220 | 0.627 (0.297–1.323) | – | – | |
| Synchronous metastasis | 0.826 | 1.130 (0.381–3.353) | – | – | |
| Diameter of metastases ≥3 cm | 0.391 | 0.751 (0.390–1.444) | – | – | |
| Multiple metastases | 0.173 | 0.613 (0.303–1.239) | – | – | |
| Bilobar liver distribution | 0.299 | 0.709 (0.370–1.358) | – | – | |
| Extrahepatic metastases | 0.895 | 0.933 (0.335–2.602) | – | – | |
| Oxaliplatin-based regimen | 0.750 | 1.119 (0.560–2.237) | – | – | |
| NAC cycles ≥4 | 0.980 | 1.009 (0.485–2.098) | – | – | |
| Targeted therapy | <0.001 | 3.778 (1.850–7.716) | <0.001 | 4.354 (1.944–9.751) | |
| Favourable clinical response | 0.001 | 3.066 (1.562–6.019) | 0.016 | 2.522 (1.188–5.353) | |
CEA, carcinoembryonic antigen; RDW, red blood cell distribution width; NAC, pre-neoadjuvant chemotherapy;
Predictive value of the pre-NAC RDW level and the preoperative RDW level on major postoperative complications
In this study, 55.3% of patients (83/150) experienced postoperative complications, including 32 major complications and 51 minor complications (surgery-related complications: 30/83, 36.1%; general complications: 37/83, 44.6%; and surgery-related and general complications: 16/83, 19.3%). The relationships between major postoperative complications and clinicopathological features are shown in Table 3. The univariate analysis revealed that a pre-NAC RDW-SD ≥42.20 fl (P=0.081), a preoperative RDW-SD ≥42.20 fl (P=0.503), a preoperative RDW-CV ≥13.5% (P=0.645) and an RDW-CV change =2 (P=0.078) were not significantly associated with major postoperative complications. In contrast, a pre-NAC RDW-CV ≥13.5% (OR =2.476, 95% CI: 1.106–5.546, P=0.028) and an RDW-SD change =2 (OR =2.471, 95% CI: 1.111–5.495, P=0.027) were significantly associated with major postoperative complications. The univariate analysis also revealed that an intraoperative blood transfusion (P=0.046), operation time ≥290 min (P=0.021) and blood loss ≥450 mL (P=0.028) were significantly associated with major postoperative complications. The multivariate analysis showed that a pre-NAC RDW-CV ≥13.5% (OR =2.462, 95% CI: 1.080–5.615, P=0.032) significantly predicted major postoperative complications and that an operation time ≥290 min (OR =3.311, 95% CI: 1.175–9.330, P=0.023) was an independent predictor of major postoperative complications.
Table 3
| Factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | ||
| Age ≥60 years | 0.842 | 1.086 (0.484–2.438) | – | – | |
| Male | 0.676 | 0.838 (0.366–1.918) | – | – | |
| BMI ≥24 kg/m2 | 0.075 | 0.486 (0.219–1.076) | – | – | |
| Comorbidity | 0.492 | 0.758 (0.343–1.673) | – | – | |
| Preoperative CEA ≥10 ng/mL | 0.287 | 0.642 (0.284–1.451) | – | – | |
| Pre-NAC RDW-SD ≥42.20 fl | 0.081 | 2.026 (0.917–4.472) | – | – | |
| Preoperative RDW-SD ≥42.20 fl | 0.503 | 1.698 (0.360–8.007) | – | – | |
| RDW-SD change =2 | 0.027 | 2.471 (1.111–5.495) | – | – | |
| Pre-NAC RDW-CV ≥13.5% | 0.028 | 2.476 (1.106–5.546) | 0.032 | 2.462 (1.080–5.615) | |
| Preoperative RDW-CV ≥13.5% | 0.645 | 0.819 (0.350–1.916) | – | – | |
| RDW-CV change =2 | 0.078 | 2.123 (0.919–4.906) | – | – | |
| Right hemicolon | 0.479 | 1.496 (0.491–4.560) | – | – | |
| Synchronous metastasis | 0.239 | 0.500 (0.158–1.584) | – | – | |
| Diameter of metastases ≥3 cm | 0.062 | 2.131 (0.962–4.722) | – | – | |
| Multiple metastases | 0.488 | 1.370 (0.563–3.336) | – | – | |
| Bilobar liver distribution | 0.570 | 1.255 (0.574–2.745) | – | – | |
| Extrahepatic metastases | 0.815 | 1.154 (0.349–3.814) | – | – | |
| Heterochronous resection | 0.757 | 1.148 (0.479–2.753) | – | – | |
| Major liver resection | 0.063 | 2.200 (0.959–5.046) | – | – | |
| Concomitant RFA | 0.682 | 1.222 (0.469–3.183) | – | – | |
| Intraoperative blood transfusion | 0.046 | 2.420 (1.015–5.768) | – | – | |
| Operation time ≥290 min | 0.021 | 3.329 (1.196–9.268) | 0.023 | 3.311 (1.175–9.330) | |
| Blood loss ≥450 mL | 0.028 | 2.545 (1.107–5.850) | – | – | |
| Oxaliplatin-based regimen | 0.135 | 0.542 (0.243–1.210) | – | – | |
| NAC cycles ≥4 | 0.118 | 2.277 (0.811–6.396) | – | – | |
| Targeted therapy | 0.573 | 1.263 (0.560–2.849) | – | – | |
CRLM, colorectal liver metastases; CEA, carcinoembryonic antigen; RDW, red blood cell distribution width; NAC, pre-neoadjuvant chemotherapy.
Predictive value of the pre-NAC RDW level and the preoperative RDW level on PFS and OS
At the time of analysis, 123 (82.0%) patients experienced disease recurrence, and 86 (57.3%) died. The median PFS was 7.2 months (95% CI: 7.1–9.5), and the median OS was 36.8 months (95% CI: 36.0–42.3). The 1- and 3-year PFS rates were 32.0% and 17.4%, respectively. The 1-, 3- and 5-year OS rates were 92.7%, 52.5% and 35.8%, respectively.
The median PFS was 10.0 months (95% CI: 4.9–15.1) in patients with a pre-NAC RDW-CV ≥13.5 and 6.5 months (95% CI: 4.1–8.9) in those with a pre-NAC RDW-CV <13.5 (P=0.006) (Figure 1A). The median PFS was 9.5 months (95% CI: 7.5–11.5) in patients with a preoperative RDW-CV ≥13.5% and 4.4 months (95% CI: 3.3–5.5) in those with a preoperative RDW-CV <13.5% (P=0.003) (Figure 1B). The median PFS was 14.0 months (95% CI: 5.4–22.6) in patients with an RDW-CV change =2 and 5.7 months (95% CI: 3.9–7.5) in those with an RDW-CV change =0 or 1 (P<0.001) (Figure 1C). The median PFS was 10.1 months (95% CI: 6.2–14.0) in patients with a pre-NAC RDW-SD ≥42.2 fl and 6.5 months (95% CI: 4.4–8.6) in those with a pre-NAC RDW-SD <42.2 fl (P=0.010) (Figure 1D). The median PFS was 7.7 months (95% CI: 5.4–10.0) in patients with a preoperative RDW-SD ≥42.2 fl and 5.1 months (95% CI: 1.8–8.4) in those with a preoperative RDW-SD <42.2 fl (P=0.299) (Figure 1E). The median PFS was 10.4 months (95% CI: 6.1–14.6) in patients with an RDW-SD change=2 and 6.0 months (95% CI: 4.0–8.0) in those with an RDW-SD change =0 or 1 (P=0.007) (Figure 1F).
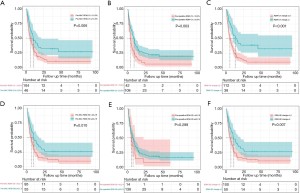
The univariate analysis revealed that a pre-NAC RDW-SD ≥42.20 fl (HR =0.606, 95% CI: 0.414–0.886, P=0.010), an RDW-SD change =2 (HR =0.585, 95% CI: 0.396–0.866, P=0.007), a pre-NAC RDW-CV ≥13.5% (HR =0.568, 95% CI: 0.379–0.853, P=0.006), a preoperative RDW-CV ≥13.5% (HR =0.558, 95% CI: 0.380–0.819, P=0.003) and an RDW-CV change =2 (HR =0.441, 95% CI: 0.281–0.692, P<0.001) were significantly associated with PFS. According to the multivariate analysis, an RDW-CV change =2 (HR =0.487, 95% CI: 0.309–0.768, P=0.002), primary lymph node metastasis (HR =2.057, 95% CI: 1.265–3.345, P=0.004), and R0 resection (HR =0.479, 95% CI: 0.328–0.699, P<0.001) remained significant for PFS (Table 4).
Table 4
| Factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | ||
| Age ≥60 years | 0.162 | 0.764 (0.523–1.114) | – | – | |
| Male | 0.942 | 0.986 (0.672–1.445) | – | – | |
| BMI ≥24 kg/m2 | 0.562 | 1.111 (0.779–1.584) | – | – | |
| Comorbidity | 0.713 | 0.935 (0.655–1.335) | – | – | |
| Preoperative CEA ≥10 ng/mL | 0.274 | 1.221 (0.853–1.748) | – | – | |
| Pre-NAC RDW-SD ≥42.20 fl | 0.010 | 0.606 (0.414–0.886) | – | – | |
| Preoperative RDW-SD ≥42.20 fl | 0.299 | 0.736 (0.414–1.311) | – | – | |
| RDW-SD change =2 | 0.007 | 0.585 (0.396–0.866) | – | – | |
| Pre-NAC RDW-CV ≥13.5% | 0.006 | 0.568 (0.379–0.853) | – | – | |
| Preoperative RDW-CV ≥13.5% | 0.003 | 0.558 (0.380–0.819) | – | – | |
| RDW-CV change =2 | 0.000 | 0.441 (0.281–0.692) | 0.002 | 0.487 (0.309–0.768) | |
| Right hemicolon | 0.135 | 0.623 (0.335–1.158) | – | – | |
| Poor differentiation | 0.286 | 1.229 (0.840–1.799) | – | – | |
| T3–T4 stage | 0.126 | 1.510 (0.891–2.557) | – | – | |
| Primary lymph node metastasis | <0.001 | 2.494 (1.551–4.011) | 0.004 | 2.057 (1.265–3.345) | |
| Synchronous metastasis | 0.862 | 1.054 (0.581–1.913) | – | – | |
| Diameter of metastases ≥3 cm | 0.788 | 1.051 (0.733–1.506) | – | – | |
| Multiple metastases | 0.001 | 2.013 (1.328–3.050) | – | – | |
| Bilobar liver distribution | 0.005 | 1.665 (1.166–2.377) | – | – | |
| Extrahepatic metastases | 0.857 | 1.051 (0.612–1.805) | – | – | |
| Heterochronous resection | 0.335 | 1.218 (0.816–1.816) | – | – | |
| R0 resection | <0.001 | 0.434 (0.299–0.629) | <0.001 | 0.479 (0.328–0.699) | |
| Major liver resection | 0.005 | 1.681 (1.171–2.414) | – | – | |
| Concomitant RFA | 0.008 | 1.789 (1.168–2.740) | – | – | |
| Operation time ≥339 min | 0.175 | 1.278 (0.897–1.821) | – | – | |
| Blood loss ≥250 mL | 0.617 | 1.095 (0.768–1.560) | – | – | |
| Intraoperative blood transfusion | 0.405 | 1.200 (0.782–1.840) | – | – | |
| Major postoperative complications | 0.858 | 1.040 (0.675–1.604) | – | – | |
| Oxaliplatin-based regimen | 0.037 | 1.256 (1.014–1.557) | – | – | |
| NAC cycles ≥4 | 0.039 | 1.554 (1.023–2.360) | – | – | |
| Targeted therapy | 0.080 | 1.390 (0.962–2.009) | – | – | |
| Postoperative chemotherapy | 0.143 | 0.766 (0.536–1.094) | – | – | |
| Favourable clinical response | 0.876 | 0.972 (0.682–1.386) | – | – | |
| Favourable pathological response | 0.014 | 0.633 (0.439–0.912) | – | – | |
PFS, progression-free survival; CRLM, colorectal liver metastases; CEA, carcinoembryonic antigen; RDW, red blood cell distribution width; NAC, pre-neoadjuvant chemotherapy.
The median OS was 41.0 months (95% CI: 29.2–42.8) in patients with a pre-NAC RDW-CV ≥13.5% and 36.0 months (95% CI: 29.2–42.8) in those with a pre-NAC RDW-CV <13.5% (P=0.436) (Figure 2A). The median OS was 40.3 months (95% CI: 32.6–48.0) in patients with a preoperative RDW-CV ≥13.5% and 36.0 months (95% CI: 22.2–49.8) in those with a preoperative RDW-CV <13.5% (P=0.358) (Figure 2B). The median OS was 42.3 months (95% CI not reached) in patients with an RDW-CV change =2 and 36.0 months (95% CI: 30.0–42.0) in those with an RDW-CV change =0 or 1 (P=0.090) (Figure 2C). The median OS was 46.0 months (95% CI: 32.4–59.6) in patients with a pre-NAC RDW-SD ≥42.2 fl and 34.6 months (95% CI: 30.5–38.7) in those with a pre-NAC RDW-SD <42.2 fl (P=0.052) (Figure 2D). The median OS was 36.8 months (95% CI: 29.6–43.9) in patients with a preoperative RDW-SD ≥42.2 fl and 32.6 months (95% CI: 14.9–50.3) in those with a preoperative RDW-SD <42.2 fl (P=0.534) (Figure 2E). The median OS was 50.0 months (95% CI: 32.9–67.1) in patients with an RDW-SD change =2 and 34.6 months (95% CI: 30.1–39.1) in those with an RDW-SD change =0 or 1 (P=0.065) (Figure 2F).
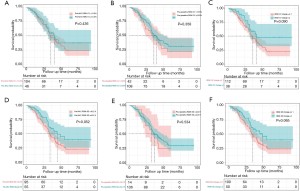
The univariate analysis also revealed that primary lymph node metastasis (P<0.001), multiple liver metastases (P=0.001), bilobar liver distribution (P=0.005), R0 resection (P<0.001), major liver resection (P=0.005), concomitant radiofrequency ablation (RFA) (P=0.008), an oxaliplatin-based regimen (P=0.037), ≥4 NAC cycles (P=0.039) and pathological response (P=0.014) were significantly associated with OS. The multivariate analysis revealed that an RDW-SD change =2 (HR =0.532, 95% CI: 0.332–0.854, P=0.009), primary lymph node metastasis (HR =2.024, 95% CI: 1.093–3.748, P=0.025) and R0 resection (HR =0.383, 95% CI: 0.246–0.597, P<0.001) were independent predictors of OS (Table 5).
Table 5
| Factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | ||
| Age ≥60 years | 0.170 | 0.724 (0.457–1.148) | – | – | |
| Male | 0.881 | 1.035 (0.656–1.633) | – | – | |
| BMI ≥24 kg/m2 | 0.194 | 1.329 (0.866–2.040) | – | – | |
| Comorbidity | 0.306 | 0.798 (0.519–1.228) | – | – | |
| Preoperative CEA ≥10 ng/mL | 0.431 | 1.186 (0.776–1.813) | – | – | |
| Pre-NAC RDW-SD ≥42.20 fl | 0.052 | 0.630 (0.396–1.005) | – | – | |
| Preoperative RDW-SD ≥42.20 fl | 0.534 | 0.811 (0.419–1.569) | – | – | |
| RDW-SD change =2 | 0.065 | 0.634 (0.390–1.029) | 0.009 | 0.532 (0.332–0.854) | |
| Pre-NAC RDW-CV ≥13.5% | 0.436 | 0.827 (0.513–1.334) | – | – | |
| Preoperative RDW-CV ≥13.5% | 0.358 | 0.806 (0.508–1.278) | – | – | |
| RDW-CV change =2 | 0.090 | 0.624 (0.362–1.076) | – | – | |
| Right hemicolon | 0.918 | 1.037 (0.519–2.075) | – | – | |
| Poor differentiation | 0.119 | 1.428 (0.912–2.235) | – | – | |
| T3–T4 stage | 0.518 | 1.233 (0.654–2.323) | – | – | |
| Primary lymph node metastasis | 0.007 | 2.324 (1.261–4.281) | 0.025 | 2.024 (1.093–3.748) | |
| Synchronous metastasis | 0.626 | 0.842 (0.421–1.682) | – | – | |
| Diameter of metastases ≥3 cm | 0.204 | 1.319 (0.861–2.020) | – | – | |
| Multiple metastases | 0.027 | 1.743 (1.064–2.855) | – | – | |
| Bilobar liver distribution | 0.070 | 1.482 (0.968–2.269) | – | – | |
| Extrahepatic metastases | 0.720 | 1.123 (0.596–2.117) | – | – | |
| Heterochronous resection | 0.053 | 1.562 (0.993–2.456) | 0.028 | 1.678 (1.058–2.660) | |
| R0 resection | <0.001 | 0.399 (0.259–0.616) | <0.001 | 0.383 (0.246–0.597) | |
| Major liver resection | 0.034 | 1.598 (1.037–2.464) | – | – | |
| Concomitant RFA | 0.148 | 1.440 (0.879–2.359) | – | – | |
| Operation time ≥339 min | 0.827 | 1.049 (0.686–1.602) | – | – | |
| Blood loss ≥250 mL | 0.360 | 1.220 (0.797–1.867) | – | – | |
| Intraoperative blood transfusion | 0.111 | 1.494 (0.912–2.447) | – | – | |
| Major postoperative complications | 0.539 | 1.187 (0.687–2.049) | – | – | |
| Oxaliplatin-based regimen | 0.085 | 0.678 (0.436–1.055) | – | – | |
| NAC cycles ≥4 | 0.163 | 1.449 (0.860–2.443) | – | – | |
| Targeted therapy | 0.614 | 1.121 (0.719–1.747) | – | – | |
| Postoperative chemotherapy | 0.052 | 0.656 (0.429–1.004) | – | – | |
| Favourable clinical response | 0.194 | 0.755 (0.493–1.154) | – | – | |
| Favourable pathological response | 0.092 | 0.687 (0.445–1.063) | – | – | |
OS, overall survival; CRLM, colorectal liver metastases; CEA, carcinoembryonic antigen; RDW, red blood cell distribution width; NAC, pre-neoadjuvant chemotherapy.
Discussion
This study revealed the predictive significance of the pre-NAC RDW level, preoperative RDW level and RDW change on the pathological response, major postoperative complications, PFS and OS in CRLM patients receiving NAC followed by liver resection: (I) a preoperative RDW-CV ≥13.5% was significantly associated with a favorable pathological response; (II) a pre-NAC RDW-CV ≥13.5% was significantly associated with the occurrence of major postoperative complications; (III) an RDW-CV change =2 was an independent predictor of favorable PFS; and (IV) an RDW-SD change =2 was an independent predictor of favorable OS.
The ability to apply markers with predictive ability before patients receive treatment has significant clinical utility since those markers can provide information about the effectiveness of treatment and tumour aggressiveness. The RDW level, which is an indicator of the degree of erythrocyte morphology imbalance in the blood, reflects the heterogeneity in the erythrocyte volume (18) and is a biomarker easily obtained from blood tests. This study first identified the ability of elevated preoperative RDW-CV to predict a favorable pathological response in CRLM patients receiving NAC. An elevated preoperative RDW level is more likely to be the result of a favorable pathological response. Previous studies have revealed that chemotherapy agents not only promote direct cytotoxicity but also elicit immune-potentiating effects by promoting lymphocyte infiltration, expressing a large number of inflammatory factors (e.g., IL-6, TNF), disrupting tumour-induced immunosuppressive networks and sensitizing tumour cells to immune attacks (27,28). In addition, the activation of inflammatory cells and inflammatory factors was found to promote an increase in immature red blood cells and a subsequent increase in RDW (29,30). Therefore, the relationship between pathological response and the RDW level can be predictable.
This study also revealed for the first time that a primary tumour site in the right hemicolon was significantly associated with a favorable pathological response. The right hemicolon is commonly considered the most immunoreactive section of the colorectum (31). Defective mismatch repair also primarily occurs in the right colon, where a strong local immune infiltration is seen in the tumour microenvironment (32). Thus, a primary tumour in the right colon with a favorable pathological response is reasonable. In addition, a significant correlation between the radiological response and the pathological response was identified, consistent with a previous study (11). This significant correlation may be attributed to the routine use of MRI for therapeutic evaluations at our hospital. It has also been revealed that the apparent diffusion coefficient of MRI is the most precise parameter for predicting the chemotherapy response in CRLM patients (33).
The occurrence of major postoperative complications in CRLM patients often leads to unfavorable outcomes, such as poor quality of life, extended recovery time, and increased economic and physical stress. The effective prediction of major postoperative complications will facilitate the formulation of protective measures and postoperative treatment plans. Some studies have shown that various intraoperative factors, such as operation time, blood transfusion and major liver resection, were associated with the increased occurrence of major postoperative complications (4,6). This study first revealed that the pre-NAC RDW-CV level significantly predicted major postoperative complications, which indicates that a long interval from NAC to liver resection could be more helpful in the adjustment of treatment plans. The reasons for this relationship are related to the following two aspects: first, the increase in RDW level is accompanied by an elevation in reactive oxygen species and tissue hypoxia, and these factors are associated with a high risk of postoperative complications (34); second, the RDW level is associated with the dysfunction of various organs (35,36). Patients with elevated RDW levels are more likely to develop organ dysfunction, which leads to postoperative complications.
Studies have revealed that the RDW level is an independent predictor of postoperative survival in patients with hepatocellular carcinoma (22), esophageal carcinoma (23) and colorectal cancer (24). A previous study focused on the impact of preoperative RDW levels on survival. This study first revealed the prognostic value of the pre-NAC RDW level, the preoperative RDW level and the RDW change in CRLM patients receiving NAC, the results of which demonstrated that an RDW change =2 was an independent predictor of postoperative favorable survival in CRLM patients, while the preoperative RDW level was not an independent prognostic factor. These results emphasize the importance of monitoring changes in RDW levels from NAC to resection in CRLM patients. Another interesting finding of this study was that the RDW-SD change was an independent predictor of OS and that the RDW-CV change was an independent predictor of PFS. RDW values are reflected in the RDW-CV levels and RDW-SD levels, but the predictive values of these two forms are different. The intrinsic mechanism needs to be further explored since the mechanisms of the relationship between the RDW level and patient outcomes are unclear. First, elevated RDW levels are closely related to an increase in cytokines, such as interleukin 1 (IL)-1, TNF, and CRP (29,30). Activation of the cytokines IL-1 and TNF leads to an increase in the number of immature red blood cells, which results in an increase in RDW levels (29,30). Cytokines, such as IL-1 and TNF, can promote M1 macrophage polarization in the tumour immune microenvironment and then exert anti-inflammatory and anti-tumour activities (37). Second, tumour cell metabolism mainly relies on sugar enzymes in the tumour microenvironment. The RDW level is considered an important marker that affects tumour cell glycolysis, and an elevated RDW level can lead to glycolysis disturbances and the inhibition of tumour progression (38).
This study has several limitations. First, this study included a limited number of patients from a single institution. Second, the KRAS status of all patients was not included because the cost of KRAS testing is high and not covered by insurance in China. Third, the results of this study may require further validation in other cohorts or in a prospective study.
In conclusion, the pre-NAC RDW level, preoperative RDW level and change in RDW level are reliable markers that can be used to predict a pathological response and prognosis in CRLM patients receiving NAC followed by liver resection, which is helpful for treatment decision-making, surveillance and prognostication.
Acknowledgments
Thanks for the AJE language service.
Funding: This study was supported by the State Key Project on Infection Diseases of China (Grant No. 2017ZX10201021-007-003), the National The capital health research and development of special (Grant No. 2018-1-4021), the National Natural Science Foundation of China (Grant Nos. 81972311), the CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant No. 2017-12M-4-002), the Non-profit Central Research Institution Fund of the Chinese Academy of Medical Sciences (Grant No. 2019PT310026) and the Sanming Project of Medicine in Shenzhen (Grant No. SZSM202011010).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-934
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-934
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-934). The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the Cancer Institute & Hospital, Chinese Academy of Medical Sciences (ID: NCC2019C-016). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent has been obtained from patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70:145-64. [Crossref] [PubMed]
- Engstrand J, Nilsson H, Strömberg C, et al. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer 2018;18:78. [Crossref] [PubMed]
- Chen Q, Zhao H, Wu J, et al. Preoperative D-dimer and Gamma-Glutamyltranspeptidase Predict Major Complications and Survival in Colorectal Liver Metastases Patients After Resection. Transl Oncol 2019;12:996-1004. [Crossref] [PubMed]
- de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 2009;250:440-8. [Crossref] [PubMed]
- Chen Q, Wu C, Zhao H, et al. Neo-adjuvant Chemotherapy-Induced Neutropenia Is Associated with Histological Responses and Outcomes after the Resection of Colorectal Liver Metastases. J Gastrointest Surg 2020;24:659-70. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [Crossref] [PubMed]
- Pietrantonio F, Mazzaferro V, Miceli R, et al. Pathological response after neoadjuvant bevacizumab- or cetuximab-based chemotherapy in resected colorectal cancer liver metastases. Med Oncol 2015;32:182. [Crossref] [PubMed]
- Berardi G, De Man M, Laurent S, et al. Radiologic and pathologic response to neoadjuvant chemotherapy predicts survival in patients undergoing the liver-first approach for synchronous colorectal liver metastases. Eur J Surg Oncol 2018;44:1069-77. [Crossref] [PubMed]
- Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol 2007;18:299-304. [Crossref] [PubMed]
- Chen Q, Mao R, Zhao J, et al. From the completion of neoadjuvant chemotherapy to surgery for colorectal cancer liver metastasis: What is the optimal timing? Cancer Med 2020;9:7849-62. [Crossref] [PubMed]
- Blazer DG 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008;26:5344-51. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208-15. [Crossref] [PubMed]
- Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol 2017;32:43-53. [Crossref] [PubMed]
- Lichtenstern CR, Ngu RK, Shalapour S, et al. Immunotherapy, Inflammation and Colorectal Cancer. Cells 2020;9:618. [Crossref] [PubMed]
- Erstad DJ, Taylor MS, Qadan M, et al. Platelet and neutrophil to lymphocyte ratios predict survival in patients with resectable colorectal liver metastases. Am J Surg 2020;220:1579-85. [Crossref] [PubMed]
- Taniai T, Haruki K, Hamura R, et al. The Prognostic Significance of C-reactive Protein-To-Lymphocyte Ratio in Colorectal Liver Metastases. J Surg Res 2021;258:414-21. [Crossref] [PubMed]
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86-105. [Crossref] [PubMed]
- Fava C, Cattazzo F, Hu ZD, et al. The role of red blood cell distribution width (RDW) in cardiovascular risk assessment: useful or hype? Ann Transl Med 2019;7:581. [Crossref] [PubMed]
- Wu J, Zhang X, Liu H, et al. RDW, NLR and RLR in predicting liver failure and prognosis in patients with hepatitis E virus infection. Clin Biochem 2019;63:24-31. [Crossref] [PubMed]
- Ai L, Mu S, Hu Y. Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Int 2018;18:61. [Crossref] [PubMed]
- Zhao T, Cui L, Li A. The significance of RDW in patients with hepatocellular carcinoma after radical resection. Cancer Biomark 2016;16:507-12. [Crossref] [PubMed]
- Wan GX, Chen P, Cai XJ, et al. Elevated red cell distribution width contributes to a poor prognosis in patients with esophageal carcinoma. Clin Chim Acta 2016;452:199-203. [Crossref] [PubMed]
- Pedrazzani C, Tripepi M, Turri G, et al. Prognostic value of red cell distribution width (RDW) in colorectal cancer. Results from a single-center cohort on 591 patients. Sci Rep 2020;10:1072. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Karagiannis GS, Condeelis JS, Oktay MH. Chemotherapy-Induced Metastasis: Molecular Mechanisms, Clinical Manifestations, Therapeutic Interventions. Cancer Res 2019;79:4567-76. [Crossref] [PubMed]
- Luo Q, Zhang L, Luo C, et al. Emerging strategies in cancer therapy combining chemotherapy with immunotherapy. Cancer Lett 2019;454:191-203. [Crossref] [PubMed]
- Vayá A, Sarnago A, Fuster O, et al. Influence of inflammatory and lipidic parameters on red blood cell distribution width in a healthy population. Clin Hemorheol Microcirc 2015;59:379-85. [Crossref] [PubMed]
- Hu L, Li M, Ding Y, et al. Prognostic value of RDW in cancers: a systematic review and meta-analysis. Oncotarget 2017;8:16027-35. [Crossref] [PubMed]
- Patel M, McSorley ST, Park JH, et al. The relationship between right-sided tumour location, tumour microenvironment, systemic inflammation, adjuvant therapy and survival in patients undergoing surgery for colon and rectal cancer. Br J Cancer 2018;118:705-12. [Crossref] [PubMed]
- Gunnarsson U, Strigård K, Edin S, et al. Association between local immune cell infiltration, mismatch repair status and systemic inflammatory response in colorectal cancer. J Transl Med 2020;18:178. [Crossref] [PubMed]
- Beckers RCJ, Lambregts DMJ, Lahaye MJ, et al. Advanced imaging to predict response to chemotherapy in colorectal liver metastases - a systematic review. HPB (Oxford) 2018;20:120-7. [Crossref] [PubMed]
- Xie DX, Rehman SC, Francis DO, et al. Association Between Red Blood Cell Distribution Width and Outcomes of Open Airway Reconstruction Surgery in Adults. JAMA Otolaryngol Head Neck Surg 2019;145:210-5. [Crossref] [PubMed]
- Lippi G, Targher G, Montagnana M, et al. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand J Clin Lab Invest 2008;68:745-8. [Crossref] [PubMed]
- Hu Z, Sun Y, Wang Q, et al. Red blood cell distribution width is a potential prognostic index for liver disease. Clin Chem Lab Med 2013;51:1403-8. [Crossref] [PubMed]
- Wu J, Li K, Peng W, et al. Autoinducer-2 of Fusobacterium nucleatum promotes macrophage M1 polarization via TNFSF9/IL-1β signaling. Int Immunopharmacol 2019;74:105724 [Crossref] [PubMed]
- Vitale I, Manic G, Coussens LM, et al. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab 2019;30:36-50. [Crossref] [PubMed]


