Effectiveness and safety of fractional micro-plasma radio-frequency treatment combined with ablative fractional carbon dioxide laser treatment for hypertrophic scar: a retrospective study
Introduction
Wound healing is a normal physiological process, however, some wounds caused by surgery, burns, and trauma are prone to heal abnormally, resulting in hypertrophic scars. Hypertrophic scars are mainly caused by the rapid proliferation of fibroblasts and the deposition of excess extracellular collagen (1). Compared with keloid, a hypertrophic scar will be limited to the initial wound site and will not exceed the original boundary (2). According to a previous study, approximately 60% of patients developed hypertrophic scars after surgical procedures (3) and some hypertrophic scars will fade over time. Hypertrophic scars caused by burns are also very common, and the injury to patients is greater due to the randomness and unpredictability of scar site. It has been reported that hypertrophic scar affects 32–72% of burn survivors based on patient age, wound infection, gender, and burn depth (4,5). The formation of hypertrophic scar can lead to unsightly appearance, restricted movement, psychological burden, and abnormal bodily sensations.
At present, the treatment of hypertrophic scar includes both surgical and non-surgical options. Non-surgical treatment is the preferred treatment, and surgical treatment is usually only suitable for the treatment of severe hypertrophic scar. Non-surgical treatments include compression therapy, silicone gels, topical 5-fluorouracil, and steroid injections (6-9). However, due to the half-life of 5-fluorouracil and steroids, they cannot maintain a long-term effect and usually require multiple applications. In addition, due to their side effects, 5-fluorouracil and steroids can only be used limitedly and locally. Silicone gel and compression therapy are more suitable for the prevention of hypertrophic scar.
Fractional micro-plasma radiofrequency technology has been developed for the treatment of hypertrophic scars in recent years. It can trigger rapid re-epithelialization in the epidermis and accelerate the remodeling of fibroblasts. It has been reported to be effective and safe for the treatment for acne scars and hypertrophic scars (10,11). Besides, ablative fractional carbon dioxide (CO2) laser treatment has also been developed for the treatment of hypertrophic scars by vaporizing water molecules and ablating scars (4). A previous study indicated that ablative fractional CO2 laser treatment was effective in reducing the thickness of scars and improving the extensibility of involved tissues (12). Recently, more and more attention has been paid to the treatment of hypertrophic scar with a combination of diverse treatments, and therapeutic effects of combined therapy have been investigated.
This study was performed retrospectively in our center to investigate the safety and effectiveness of fractional micro-plasma radio-frequency treatment combined with ablative fractional CO2 laser treatment in patients with hypertrophic scars. The hypothesis was proposed that the combination of the two methods could effectively remove hypertrophic scars, and would not significantly increase the incidence of adverse complications. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-2153).
Methods
Patient selection
This study was a retrospective study performed in a single center of the Affiliated Hospital of Jiangnan University between January 2019 and December 2020. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Hospital of Jiangnan University (No.: 2021081). Individual consent for this retrospective analysis was waived.
Patients with hypertrophic scars due to diverse etiologies were all enrolled in this study. The inclusion criteria encompassed patients who were older than 18 years, younger than 70 years, suffering from serious underlying disease, receiving fractional micro-plasma radio-frequency treatment or ablative fractional CO2 laser treatment, or combined therapy of both. The following patients were excluded from this study: those younger than 18 years old, older than 70 years old, not receiving either fractional micro-plasma radio-frequency treatment or ablative fractional CO2 laser treatment, and with incomplete data.
According to the treatments they received, participants were divided into a single treatment and a combined treatments group. Some participants data were collected, including age, gender, etiology of scars, location of scars, time of scar formation, number of treatments, and time between treatments.
Fractional micro-plasma radio-frequency treatment
Fractional micro-plasma radio-frequency treatment was performed using a plasma beam scar therapy apparatus with a roller head (Alma Lasers, Caesarea, Israel) at a power of 60–70 W and a current of 4–6 mA.
Ablative fractional CO2 laser treatment
Ablative fractional CO2 laser treatment was performed using an ablative fractional CO2 laser (Lumenis, Yokneam, Israel). The settings were as follows: density of 5–10%, energy of 15–30 MJ, and a frequency of 300 Hz. The size of light spot and the dose of laser varied based on the size and thickness of scars. The interval between the two treatments was typically about 2–3 months.
Assessments of effectiveness and safety
Participants received outpatient follow-up and the mean duration of follow up was 7.2±2.2 months. The effectiveness of scar treatment was assessed by the score of the Vancouver scar scale and the changes of scar thickness. The Vancouver scar scale contains four items, including pigmentation (0–3 points), vascularity (0–3 points), pliability (0–5 points), and height (0–4 points), with a total score of 15 points. The thickness of scars was measured using a high-frequency ultrasound device. Some adverse complications were also recorded to evaluate the safety of treatments, such as pruritus, pain, seepage, bleeding, and swelling.
Subgroup analysis
A subgroup analysis was performed to investigate the differences in effectiveness and safety of combined therapy in scar patients at different stages. Participants who received treatment within 6 months postinjury were defined as early stage while those receiving treatment more than 6 months postinjury were defined as late stage. Then, the differences of effectiveness and safety of combined therapy in these subgroups were analyzed.
Statistical analysis
The statistical analysis in this study was performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Continuous variables were reported as mean with standard deviation (SD) and analyzed using two-tailed Mann-Whitney U test for comparisons between two groups or subgroups. Categorical variables were reported as numbers and percentages and analyzed using chi-square (χ2) tests. A P value less than 0.05 was considered statistically significant.
Results
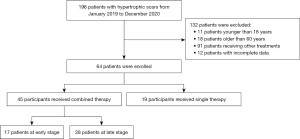
There were 196 patients admitted to our center for the treatment of hypertrophic scars between January 2019 and December 2020 (Figure 1). Among them, 132 patients were eliminated based on the exclusion criteria. Finally, 64 patients with hypertrophic scars were enrolled in this study, including 45 who received fractional micro-plasma radio-frequency treatment combined with ablative fractional CO2 laser treatment, and 19 who received either treatment singly.
Participant demographic data are listed in Table 1. The mean age in the single therapy group was 35.2±11.3 years, and that in combined therapy group was 34.6±13.6 years. More than two-thirds of participants in both groups were male and the most common etiology of hypertrophic scars in this study was burn. The most common location of scars in both groups was head and/or neck, followed by extremity and trunk. The time of scar formation was 8.1±2.0 months in the single therapy group and 7.4±2.6 months in the combined therapy group. A total of 10 participants received only 1 treatment in the single therapy group, 4 participants received 2 treatments, and 5 received more than 2 treatments. In the combined therapy group, 19 participants received 1 treatment, 16 received 2 treatments, and 10 received more than 2 treatments. The interval between treatments was 2.4±0.7 months in the single therapy group and 2.1±0.9 months in the combined therapy group. Generally, there was no significant difference in demographic data between the two groups.
Table 1
| Demographics | Single therapy | Combined therapy | P value |
|---|---|---|---|
| Number | 19 | 45 | |
| Age, year | 35.2±11.3 | 34.6±13.6 | 0.090 |
| Gender, n (%) | 0.690 | ||
| Male | 13 (68.4) | 33 (73.3) | |
| Female | 6 (31.6) | 12 (26.7) | |
| Etiology of scars, n (%) | 0.828 | ||
| Burn | 11 (57.9) | 31 (68.9) | |
| Surgical procedures | 3 (15.8) | 6 (13.3) | |
| Chemical agent | 2 (10.5) | 5 (11.1) | |
| Electricity | 2 (10.5) | 2 (4.4) | |
| Other | 1 (5.3) | 1 (2.2) | |
| Location of scars, n (%) | 0.955 | ||
| Head and/or neck | 9 (47.4) | 20 (44.4) | |
| Trunk | 4 (21.1) | 11 (24.4) | |
| Extremity | 6 (31.6) | 14 (31.1) | |
| Time of scar formation, month | 8.1±2.0 | 7.4±2.6 | 0.525 |
| Number of treatments, n (%) | 0.518 | ||
| 1 | 10 (52.6) | 19 (42.2) | |
| 2 | 4 (21.1) | 16 (35.6) | |
| >2 | 5 (26.3) | 10 (22.2) | |
| Interval between treatments, month | 2.4±0.7 | 2.1±0.9 | 0.159 |
The effectiveness of single therapy and combined therapy were assessed using the score of Vancouver scar scale and the changes of scar thickness, as summarized in Table 2. Both single therapy and combined therapy could significantly improve hypertrophic scars in participants according to the score of Vancouver scar scale (P=0.044 and P<0.001). Notably, combined therapy could more effectively improve hypertrophic scars and reduce the score of Vancouver scar scale compared with single therapy (P=0.026), although no other significant difference was found in the comparisons of four detailed items. In addition, both therapies could dramatically reduce the thickness of scars (both P<0.001); however, no significant difference was observed between the two groups.
Table 2
| Demographics | Single therapy | Combined therapy | P valueb |
|---|---|---|---|
| Total score of Vancouver scar scale | |||
| Before | 8.5±2.0 | 8.9±1.5 | 0.617 |
| After | 7.5±1.8 | 7.5±1.5 | 0.845 |
| P valuea | 0.044 | <0.001 | |
| Change | 0.9±0.5 | 1.4±0.7 | 0.026 |
| Pigmentation | |||
| Before | 1.9±0.7 | 2.0±0.6 | 0.464 |
| After | 1.6±0.5 | 1.6±0.6 | 0.932 |
| P valuea | 0.047 | 0.018 | |
| Change | 0.3±0.5 | 0.4±0.5 | 0.416 |
| Vascularity | |||
| Before | 1.7±0.7 | 1.7±0.7 | 0.786 |
| After | 1.5±0.5 | 1.6±0.5 | 0.833 |
| P valuea | 0.200 | 0.520 | |
| Change | 0.2±0.4 | 0.2±0.4 | 0.850 |
| Pliability | |||
| Before | 2.1±0.6 | 2.2±0.6 | 0.471 |
| After | 1.8±0.6 | 1.8±0.7 | 0.909 |
| P valuea | 0.051 | 0.033 | |
| Change | 0.3±0.5 | 0.4±0.5 | 0.305 |
| Height | |||
| Before | 2.8±0.7 | 2.9±0.9 | 0.659 |
| After | 2.5±0.6 | 2.5±0.7 | 0.937 |
| P valuea | 0.087 | 0.048 | |
| Change | 0.3±0.6 | 0.4±0.5 | 0.558 |
| Scar thickness, mm | |||
| Before | 6.7±1.9 | 6.8±2.4 | 0.923 |
| After | 3.1±1.0 | 3.0±0.8 | 0.553 |
| P valuea | <0.001 | <0.001 | |
| Change | 3.6±0.9 | 3.8±1.0 | 0.455 |
a, comparison between the score of Vancouver scar scale before and after the treatment; b, comparison between the score of Vancouver scar scale in different groups.
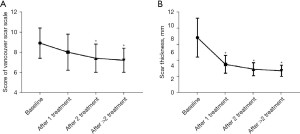
The impacts of number of treatments on the effectiveness of combined therapy was then assessed (Figure 2). It was found that multiple treatments could further increase the effectiveness of combined therapy in improving hypertrophic scars, according to either the score of Vancouver scar scale or the thickness of scars.
Adverse complication data were collected to assess the safety of combined therapy (Table 3). The incidence of total adverse complications was 71.1% (32 cases) and 68.4% (13 cases), in the single therapy and combined therapy groups, respectively, showing no significant difference between them. The most common complication in both groups was pruritus, followed by seepage, and bleeding.
Table 3
| Demographics | Single therapy, n (%) | Combined therapy, n (%) | P value |
|---|---|---|---|
| Pruritus | 4 (21.1) | 12 (26.7) | 0.599 |
| Pain | 1 (5.3) | 3 (6.7) | 0.659 |
| Seepage | 4 (21.1) | 7 (15.6) | 0.420 |
| Bleeding | 3 (15.8) | 7 (15.6) | 0.624 |
| Swelling | 1 (5.3) | 4 (8.9) | 0.532 |
| Total | 13 (68.4) | 32 (71.1) | 0.891 |
Next, participants in the combined therapy group were divided into two subgroups, an early stage subgroup and a late stage subgroup. Generally, there was no significant difference between the two groups, as shown in Table 4, except the time of scar formation. The effectiveness of combined therapy in different subgroups was assessed according to the score of Vancouver scar scale and the changes of scar thickness (Table 5). Combined therapy was found to be significantly effective at improving hypertrophic scars in patients at the early stage or late stage. More importantly, combined therapy could reduce the score of Vancouver scar scale by 1.7±0.7 points in patients at the early stage, much more than the 1.3±0.6 points in patients at the late stage (P=0.032). Similarly, combined therapy could reduce scar thickness by 4.2±0.8 mm in patients at the early stage, much more than the 3.7±0.9 mm in patients at the late stage (P=0.042).
Table 4
| Demographics | Early stage | Late stage | P value |
|---|---|---|---|
| Number | 17 | 28 | |
| Age, year | 34.0±10.4 | 34.8±13.5 | 0.237 |
| Gender, n (%) | 0.496 | ||
| Male | 13 (76.5) | 20 (71.4) | |
| Female | 4 (23.5) | 8 (28.6) | |
| Etiology of scars, n (%) | 0.375 | ||
| Burn | 13 (76.5) | 18 (64.3) | |
| Surgical procedures | 1 (5.9) | 5 (17.9) | |
| Chemical agent | 2 (11.8) | 3 (10.7) | |
| Electricity | 0 (0.0) | 2 (7.1) | |
| Other | 1 (5.9) | 0 (0.0) | |
| Location of scars, n (%) | 0.318 | ||
| Head and/or neck | 10 (58.9) | 10 (35.7) | |
| Trunk | 3 (17.6) | 8 (28.6) | |
| Extremity | 4 (23.5) | 10 (35.7) | |
| Time of scar formation, month | 4.6±1.5 | 9.0±1.6 | <0.001 |
| Number of treatments, n (%) | 0.524 | ||
| 1 | 9 (52.9) | 10 (35.7) | |
| 2 | 5 (29.4) | 11 (39.3) | |
| >2 | 3 (17.6) | 7 (25.0) | |
| Interval between treatments, month | 2.2±0.8 | 2.1±1.1 | 0.105 |
Table 5
| Demographics | Early stage | Late stage | P valueb |
|---|---|---|---|
| Total score of Vancouver scar scale | |||
| Before | 8.8±1.3 | 9.1±1.6 | 0.517 |
| After | 7.1±1.3 | 7.8±1.5 | 0.118 |
| P valuea | <0.001 | 0.003 | |
| Change | 1.7±0.7 | 1.3±0.6 | 0.032 |
| Scar thickness, mm | |||
| Before | 7.0±2.3 | 6.8±2.4 | 0.891 |
| After | 2.8±0.8 | 3.1±0.9 | 0.140 |
| P valuea | <0.001 | <0.001 | |
| Change | 4.2±0.8 | 3.7±0.9 | 0.042 |
a, comparison between the score of Vancouver scar scale before and after the treatment; b, comparison between the score of Vancouver scar scale in different groups.
Discussion
To our knowledge, this is the first study to date investigate the effectiveness and safety of fractional micro-plasma radio-frequency treatment combined with ablative fractional CO2 laser treatment in patients with hypertrophic scarring. It was found that combined therapy could more effectively improve hypertrophic scars than single treatment therapy. The incidence of adverse complications was similar between the combined and single therapy groups. A further subgroup analysis was also performed which revealed that patients at the early stage could experience more significant improvement after the treatment of combined therapy.
Ablative fractional CO2 laser technology has been employed for many years; however, it is only in recent years that this technique has been applied to the treatment of hypertrophic scars (3,4,13-19). Several case reports firstly reported the effectiveness of ablative fractional CO2 laser treatment for hypertrophic scars (20,21), then Makboul et al. enrolled 40 patients with hypertrophic scars and confirmed ablative fractional CO2 laser treatment as a feasible management for hypertrophic scarring (22). Over the recent years, combined therapies of some other treatments and ablative fractional CO2 laser treatment have also been developed to increase the effectiveness in improving hypertrophic scars. Huang et al. combined ablative fractional CO2 laser treatment with 5-fluorouracil ethosomal gel treatment in a rabbit model; however, it was found that combined therapy was not superior to single therapy of ablative fractional CO2 laser treatment (17). Zhang et al. found in their rabbit model that artesunate combined with ablative fractional CO2 laser effectively reduces hypertrophic scarring (14). Ablative fractional CO2 laser combined with a variety of laser therapy, such as 595-nm pulsed dye laser, can also better increase the effectiveness of the treatment of hypertrophic scars (15).
Fractional micro-plasma radio-frequency technology was developed before 2008 and initially used for the treatment of facial scars and rhytids in 2010 (23,24). It was subsequently used for the treatment of post-burn facial hyperpigmentation, abdominal striae, and atrophic acne scar, and provided a promising noninvasive treatment (11,25,26). Fractional micro-plasma radio-frequency treatment also was also shown to enhance the improvement of hypertrophic scars (27). A rabbit model indicated that fractional micro-plasma radio-frequency treatment could improve the color and texture and reduce microvessels in the scar tissue by reducing the level of interleukin-8 and macrophage chemoattractant protein-1 (11). It has previously been shown that fractional micro-plasma radio-frequency treatment combined with triamcinolone could improve the effectiveness of hypertrophic scar reduction (27).
Previously, there have been no reports about the combination of fractional micro-plasma radio-frequency and ablative fractional CO2 laser in the treatment of skin diseases. In this study, we found that multiple treatments significantly increased the effectiveness of combined treatment, which was similar to the findings of a previous study. Kemp Bohan et al. reported that two treatments were required to achieve a significant reduction of scar thickness when using ablative fractional CO2 laser for the treatment of hypertrophic burn scar (4). This may explain why no significant difference was observed in scar thickness between the two groups. Also, we found that patients with early stage hypertrophic scars could achieve better effectiveness after the treatment of combined therapy. Tan et al. also reported in their study that patients receiving treatments within 1 month postinjury were the most optimal patients for laser treatments (13). It was also found that patients receiving treatments more than 12 months postinjury may benefit from laser treatment (13). However, most patients in our study received treatments within 12 months after injury and this conclusion cannot be verified. The most important precaution of combined treatment in this study was to closely monitor the occurrence of any complication or adverse event in patients and take remedial measurements in time.
The limitations in this study should be noted. Firstly, this study was performed in a single center, so the sample size in this study was not large enough to further analyze the effectiveness of combined therapy using regression analysis. Some potential factors may have affected the final results in this study. A multicenter study would compensate for this limitation. Secondly, hypertrophic scars were caused by different etiologies in this study. Different etiologies may also affect the effectiveness of combined or single therapies. However, the inclusion of hyperplastic scars with only 1 etiology would have led to a serious shortage of sample size. A larger sample size would better enable the investigation of the effectiveness of combined therapy in improving hypertrophic scar caused by a certain etiology. Thirdly, most participants received treatment within 12 months postinjury. Therefore, early stage was defined as within 6 months postinjury. Different definitions may also affect the effectiveness of combined therapy but so far it has not been unified.
In conclusion, this study confirmed that combined therapy was more effective in improving hypertrophic scars than single therapy without significantly increasing the incidence of adverse complications. Furthermore, multiple treatments can be recommended for patients with severe hypertrophic scars to further improve the effectiveness and patients should be encouraged to receive treatment at an early stage.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-2153
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-2153
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-2153). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Hospital of Jiangnan University (No.: 2021081). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feng Y, Wu JJ, Sun ZL, et al. Targeted apoptosis of myofibroblasts by elesclomol inhibits hypertrophic scar formation. EBioMedicine 2020;54:102715 [Crossref] [PubMed]
- Zhang L, Qin H, Wu Z, et al. Identification of the potential targets for keloid and hypertrophic scar prevention. J Dermatolog Treat 2018;29:600-5. [Crossref] [PubMed]
- Zhang Z, Chen J, Huang J, et al. Experimental study of 5-fluorouracil encapsulated ethosomes combined with CO2 fractional laser to treat hypertrophic scar. Nanoscale Res Lett 2018;13:26. [Crossref] [PubMed]
- Kemp Bohan PM, Cooper LE, Lu KN, et al. Fractionated ablative carbon dioxide laser therapy decreases ultrasound thickness of hypertrophic burn scar: a prospective process improvement initiative. Ann Plast Surg 2021;86:273-8. [Crossref] [PubMed]
- Nedelec B, LaSalle L, de Oliveira A, et al. Within-patient, single-blinded, randomized controlled clinical trial to evaluate the efficacy of triamcinolone acetonide injections for the treatment of hypertrophic scar in adult burn survivors. J Burn Care Res 2020;41:761-9. [Crossref] [PubMed]
- Tejiram S, Zhang J, Travis TE, et al. Compression therapy affects collagen type balance in hypertrophic scar. J Surg Res 2016;201:299-305. [Crossref] [PubMed]
- Surakunprapha P, Winaikosol K, Chowchuen B, et al. A prospective randomized double-blind study of silicone gel plus herbal extracts versus placebo in pre-sternal hypertrophic scar prevention and amelioration. Heliyon 2020;6:e03883 [Crossref] [PubMed]
- Yang B, Dong Y, Shen Y, et al. Bilayer dissolving microneedle array containing 5-fluorouracil and triamcinolone with biphasic release profile for hypertrophic scar therapy. Bioact Mater 2021;6:2400-11. [Crossref] [PubMed]
- Manuskiatti W, Kaewkes A, Yan C, et al. Hypertrophic scar outcomes in fractional laser monotherapy versus fractional laser-assisted topical corticosteroid delivery: a randomized clinical trial. Acta Derm Venereol 2021;101:adv00416 [Crossref] [PubMed]
- Lan T, Xiao Y, Tang L, et al. Treatment of atrophic acne scarring with fractional micro-plasma radio-frequency in Chinese patients: a prospective study. Lasers Surg Med 2018;50:844-50. [Crossref] [PubMed]
- Zhang W, Cheng X, Li H, et al. Evaluation of the therapeutic effect of micro-plasma radio frequency on hypertrophic scars in rabbit ears. Lasers Med Sci 2018;33:1961-8. [Crossref] [PubMed]
- Issa MC, Kassuga LE, Chevrand NS, et al. Topical delivery of triamcinolone via skin pretreated with ablative radiofrequency: a new method in hypertrophic scar treatment. Int J Dermatol 2013;52:367-70. [Crossref] [PubMed]
- Tan J, Zhou J, Huang L, et al. Hypertrophic scar improvement by early intervention with ablative fractional carbon dioxide laser treatment. Lasers Surg Med 2021;53:450-7. [Crossref] [PubMed]
- Zhang J, Zhou S, Xia Z, et al. Effectiveness of artesunate combined with fractional CO2 laser in a hypertrophic scar model with underlying mechanism. Burns 2021; Epub ahead of print. [Crossref] [PubMed]
- Zhang J, Zhou S, Xia Z, et al. 595-nm pulsed dye laser combined with fractional CO2 laser reduces hypertrophic scar through down-regulating TGFβ1 and PCNA. Lasers Med Sci 2021; Epub ahead of print. [Crossref] [PubMed]
- Zhang J, Xia Z, Zhou S, et al. Effect of artesunate combined with fractional CO2 laser on the hypertrophic scar in a rabbit model. Lasers Surg Med 2021; Epub ahead of print. [Crossref] [PubMed]
- Huang J, Chen J, Wo Y, et al. CO2 fractional laser combined with 5-fluorouracil ethosomal gel treatment of hypertrophic scar macro-, microscopic, and molecular mechanism of action in a rabbit animal model. Rejuvenation Res 2021;24:131-8. [Crossref] [PubMed]
- Zhao P, Jin M, He N, et al. Clinical observation of ultrapulse CO2 dot array laser combined with botulinum toxin type A injection in the treatment of hypertrophic scar. Panminerva Med 2020; Epub ahead of print. [Crossref] [PubMed]
- Shao PL, Liao JD, Wong TW, et al. Enhancement of wound healing by non-thermal N2/Ar micro-plasma exposure in mice with fractional-CO2-laser-induced wounds. PLoS One 2016;11:e0156699 [Crossref] [PubMed]
- Lee SJ, Kim JH, Lee SE, et al. Hypertrophic scarring after burn scar treatment with a 10,600-nm carbon dioxide fractional laser. Dermatol Surg 2011;37:1168-72. [Crossref] [PubMed]
- Lee DK, Serkin AL. Carbon dioxide laser and Apligraf for a painful plantar hypertrophic scar. J Am Podiatr Med Assoc 2004;94:61-4. [Crossref] [PubMed]
- Makboul M, Makboul R, Abdelhafez AH, et al. Evaluation of the effect of fractional CO2 laser on histopathological picture and TGF-β1 expression in hypertrophic scar. J Cosmet Dermatol 2014;13:169-79. [Crossref] [PubMed]
- Halachmi S, Orenstein A, Meneghel T, et al. A novel fractional micro-plasma radio-frequency technology for the treatment of facial scars and rhytids: a pilot study. J Cosmet Laser Ther 2010;12:208-12. [Crossref] [PubMed]
- Vautz W, Michels A, Franzke J. Micro-plasma: a novel ionisation source for ion mobility spectrometry. Anal Bioanal Chem 2008;391:2609-15. [Crossref] [PubMed]
- Wang LZ, Ding JP, Yang MY, et al. Treatment of facial post-burn hyperpigmentation using micro-plasma radiofrequency technology. Lasers Med Sci 2015;30:241-5. [Crossref] [PubMed]
- Mishra V, Miller L, Alsaad SM, et al. The use of a fractional ablative micro-plasma radiofrequency device in treatment of striae. J Drugs Dermatol 2015;14:1205-8. [PubMed]
- Yu S, Li H. Microplasma radiofrequency technology combined with triamcinolone improved the therapeutic effect on Chinese patients with hypertrophic scar and reduced the risk of tissue atrophy. Ther Clin Risk Manag 2016;12:743-7. [Crossref] [PubMed]
(English Language Editor: J. Jones)