
A systematic review and meta-analysis on the effects of the ultra-pulse CO2 fractional laser in the treatment of depressed acne scars
Introduction
Acne is a common and frequently occurring disease in adolescents. It is related to the chronic inflammation of sebaceous glands of the hair follicles, and may be caused by propionibacterium acne infection, the sebaceous glands of the hair follicles, hyperkeratosis, increased sebaceous glands, or androgen secretion (1). It mainly occurs on the face, neck, front chest, and back. Severely inflamed acne may lead to the formation of acne scars. The incidence of acne scars in teenagers has reached 20% (2).
Scars can be divided into depressed scars, hyperplastic scars, and keloids, among which depressed scars are the most common type of acne scars (3). Facial acne scars not only affect the appearance, but also have a significant effect on the life and psychology of patients. Acne depressed scars are caused by a loss of collagen during the healing process of acne, which may be related to the severity of the inflammation of the acne skin lesions, and a failure to treat them in time (4). Depressed scars are initially associated with erythema, followed by hyperpigmentation and fibrosis over time (5). Related studies have reported that depressed scars may also be caused by the replacement of collagen fibers (6). Erythema and pigmentation after acne inflammation are usually temporary, but the depressed scars caused by collagen destruction are permanent. Different methods are used to classify acne depressed scars, which are classified by shape as ice-cone type, truck box type, rolling type, or mixed type (7). Relevant studies abroad have shown that different laser parameters and operating methods may have certain correlations with the curative effect of different forms of acne depressed scars (8).
There are many treatments for acne depressed scars, such as laser treatments, micro-needle treatments, injection filling, chemical exfoliation, microdermabrasion, and fat tissue transplantation (9). The laser treatment of acne depressed scars is based on a photothermal action that stimulates the growth of dermal collagen and the remodeling of tissue structure (10). Among the laser treatments, the fractional laser acts on the skin surface by emitting array-like microbeams, and the water-containing tissue of the skin absorbs this energy, and multiple micro treatment zones (MTZs) form. Under this method, the normal tissues around each MTZ are not exposed to light and heat. The epidermal cells repair quickly through cell migration, and the skin tissues heal quickly (11). The imported lattice carbon dioxide (CO2) laser has deep and active modes. The deep mode has an energy density range of 5–30 mJ, a coverage rate of 1–25%, a spot diameter of about 0.12 mm, and a depth of action of 100–3,500 µm (12). The deep mode uses “low energy density, low coverage, small spot” treatment to treat local skin lesions with high energy (12). The depth can reach the dermis layer to stimulate the proliferation of collagen, but its coverage rate is low, and the improvement of the superficial layer of the epidermis is poor. Conversely, the active mode has an energy density range of 80–100 J/cm2, a coverage rate of 55–100%, a spot diameter of about 1.33 mm, and an action depth of 10–300 µm. The active mode uses “high energy density, high coverage, large spot” treatment to superficially grind the epidermis to promote epidermal renewal, and also stimulates the growth factors secreted by keratinocytes to repair the thermal damage after the lasering (13). Ultra-pulsed CO2 fractional laser can improve facial wrinkles, skin elasticity (14), photoaging of face, neck, and limbs, shrink pores, and skin texture (15), and remove pigmented diseases, including freckles, sun spots, age spots, pigmentation, and melasma (16). Ultra-pulse CO2 fractional laser can not only remove or reduce various paralysis marks, such as acne marks, trauma marks, burn fatigue marks, skin graft paralysis marks, stretch marks, and tension marks (17). It can also treat rosacea, capillary hyperplasia and other vascular diseases such as Sivat's heterochromia, colloid milia, tattoo, eyelid xanthoma, tuberous sclerosis, pigmented hairy epidermal nevus, seborrheic keratosis etc. (18). In addition, cosmetic results can be achieved by fractional laser. Ultra-pulse CO2 fractional laser treatment is to use the laser to evenly create tiny pores on the affected area of the skin, causing a series of skin biochemical reactions, which has the effect of firming, rejuvenating, and removing pigmentation (19). Good results have been achieved in patients with depressed scars. The newly drilled holes will not overlap each other, and fractional laser treatment will only cover part of the skin tissue, and normal skin will be preserved, speeding up recovery (20). It can stimulate the proliferation of collagen fibers at the base, grind and remove the sharp edges of concave scars, flattens the concave part, remove and alleviate concave scars, accelerate the healing speed (21), and improve the treatment effect. Ultra-pulse CO2 fractional laser has the characteristics of less trauma, fast healing, less complications, less adverse reactions, and good curative effect in the treatment of pitted acne scars (22).
At present, many clinical studies have confirmed the efficacy of the fractional CO2 laser in the treatment of acne depressed scars (23,24). There are many studies on the use of the ultra-pulse CO2 fractional laser in the treatment of depressed acne scars, but no systematic evaluation of its treatment effect and safety has been conducted. In this study, a meta-analysis and systematic evaluation were conducted on the use of the ultra-pulse CO2 fractional laser in the treatment of depressed acne scars to objectively evaluate its clinical efficacy and safety. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-70/rc).
Methods
Strategies for articles retrieval
Based on the Cochrane Intervention System Review Manual, a comprehensive and systematic review was conducted. All the articles in the study included patients treated for depressed acne scars. The following keywords and medical titles were used in the specific search process: “ultra-pulse CO2 fractional laser”, “depressed acne scars”, and “acne” or “depressed scars”. The included articles examined the treatment of depressed acne scars.
Inclusion and exclusion criteria for the articles
To be eligible for inclusion in the meta-analysis, the articles had to meet the following inclusion criteria: (I) include research objects with depressed scars who had been diagnosed with acne by a physician; (II) be a double-blind randomized controlled trial (RCT); (III) use a fractional laser for patients in the experimental group, and laser treatment or other treatments different to that used in experimental group, or no treatment for those in the control group; and (IV) have complete experimental data, an accurate sample size, and complete inter-group data.
Articles were excluded if they met any of the following exclusion criteria: (I) were inconsistent with the objective of this study and could not be used; (II) included no control group, or the treatment method of the control group was unknown; (III) had incomplete original data and/or inconsistent data; (IV) was a retrospective study, review, or case report; and/or (V) was a duplicate or reprint.
Articles screening
Two researchers first independently screened the titles and abstracts according to the eligibility criteria, and conducted data extraction and quality evaluations. When the evaluation results were inconsistent, other researchers were consulted to resolve any differences based on original data in the articles. When the titles and abstracts met the criteria, the full texts were searched for data extraction. NoteExpress 2.0 was used to manage and delete duplicate articles. The included articles were checked according to the inclusion and exclusion criteria mentioned above.
The 2 researchers independently extracted relevant information from all the eligible articles, including the first author, year of publication, sample size, age, country, and gender. If any data were missing, the researchers tried to contact the authors of the original articles via email to obtain the relevant data. In relation to unavailable data, the approach in the Cochrane Evaluation Manual was applied for related conversions (e.g., the calculation of standard deviations for continuous data).
Quality evaluation
2 professionals strictly followed the 7 evaluation criteria for RCTs to repeatedly evaluate the risk of bias of the articles included in the meta-analysis. Any differences were resolved through discussion. The quality evaluation was carried out according to the “bias-risk assessment” recommended by the Cochrane System Review Manual, version 5.3. Each evaluation considered the following 7 items: (I) the use of the random method; (II) the use of allocation hiding; (II) the implementation of the blind method between patients and researchers; (IV) the effect of the blind method; (V) the completeness of the results; (VI) the credibility of the survey results; and (VII) the presence of other biases. In relation to item 7, “satisfied” indicted that the bias was relatively small, “not satisfied” meant that the bias was high, and the research did not include sufficient details, and “unknown” meant that no mention of risk was made. Methods included in the quality assessment of the literature included random sequence generation, allocation concealment, blinding, and tracking/exit. A score of 1–3 points indicated low quality, and a score of 4–7 indicated high quality.
Data extraction
The forest charts clearly show the research results of each article, and the corresponding confidence intervals (CIs). A lack of overlap between the CIs for the results of each article indicated statistical inhomogeneity among the articles. In the case of acceptable inhomogeneity, the combination of the random-effects model (REM) and the fixed-effects model (FEM) required further subgroup analyses. The subgroups were set according to different designs, and the different natures of the impact size of each subgroup were then investigated. If different properties could not be addressed and the inhomogeneity among the articles could not be ignored, the combined statistical model was adopted. The sensitivity analysis of the research results was carried out by investigating whether a single article affected the overall results of the combination. Each article included in the study was removed one at a time, the results of the remaining articles were combined, and the combined results were compared with the respective results to confirm whether the results were the same. Generally, was believed that would have an effect on comprehensive research in the following 2 situations. First, if an article was deleted, and the estimated value of the comprehensive combined effect was other than 95% of the combined effect, the results were considered significantly different. Second, if there was little difference in the results of an article that affected the whole research, indicated the sensitivity of the combined results was poor, and the results were considered unstable. If it was contrary to the above two cases, it meant the results showed sensitivity and stability was good, and the conclusion was correct.
Statistical analysis
For the systematic review, we used Review Manager 5.3 software (provided by Cochrane Collaboration) for the data processing, and the inspection level was 0.05. An I2<50% and a P>0.05 indicated no statistical heterogeneity among the articles, and a FEM was used for the meta-analysis. Conversely, an I2 ≥50% and a P≤0.05 indicated statistical heterogeneity among the articles, and a REM was used for the meta-analysis. The combined effect size of the 2 sets of the evaluation index data was the odds ratio (OR) value and its 95% CI. A forest chart was drawn based on the integrated system evaluation results to show the research conclusions. For results with high heterogeneity, the article-by-article elimination method was used to analyze the possible sources of heterogeneity, and a sensitivity analysis of the results was conducted. If the number of RCTs for a certain indicator was ≥5, publication bias had to be assessed, and the funnel chart method was used.
Results
Results of articles retrieval
A total of 636 related articles were retrieved in this study, of which 423 were retrieved from the PubMed database, 115 were retrieved from the EMbase database, and 98 were retrieved from the Ovid-Medline database. 456 duplicate articles were removed. After the titles and abstracts were read, 154 articles that obviously did not meet the inclusion criteria were excluded. After the full texts were read, 8 additional articles were eliminated. Further, 8 non-randomized controlled experiments and 4 literatures with non-required diseases were excluded. Ultimately, 6 articles that met the inclusion criteria were included in the meta-analysis (25-30). The document screening process is shown in Figure 1. The basic information of the included literature is shown in Table 1.
Table 1
| First author and year of publication | Number of cases | Intervention | Outcome indicator | |||
|---|---|---|---|---|---|---|
| Experimental group | Control group | Experimental group | Control group | |||
| Osman 2017 (25) | 30 | 30 | CO2 fractional laser | Micro-needling treatment, once a month, 5 times in total | Efficient | |
| Ahmed 2014 (26) | 14 | 14 | CO2 fractional laser | CROSS, once every 3 weeks, 4 times in total | Four-level rating | |
| Chae 2015 (27) | 20 | 20 | CO2 fractional laser | Lattice radio frequency micro-needling, once every 4 weeks, 3 times in total | Efficient | |
| Hedelund 2012 (28) | 13 | 13 | CO2 fractional laser | Lattice radio frequency treatments, once every 6 weeks, 3 times in total | Four-level rating | |
| Faghihi 2016 (29) | 16 | 16 | CO2 fractional laser | Lattice radio frequency treatments, 1 time in total | Efficient | |
| Politano 2019 (30) | 24 | 24 | CO2 fractional laser, once every 2–3 months, 3 times in a row | Micro-needling treatment, once every 2 months, 3 times in a row | Efficient | |
CO2, carbon dioxide.
Bias-risk evaluation of the included articles
The Cochrane Handbook version 5.3 systematic review writing manual was adopted to evaluate the risk of bias of the 6 articles included in this study and output the bias-risk charts (see Figures 2,3). The Jadad scale was used to evaluate the quality of each included article (see Table 2). The results indicated that each of the 6 articles included in the study had a low risk of bias, and thus these articles met the requirements for the subsequent analysis.
Table 2
| First author | Randomization | Binding | Allocation concealment | Withdrawals and dropouts | Reason of dropouts and withdrawals | Jadad |
|---|---|---|---|---|---|---|
| Osman 2017 (25) | Yes | No | NMT | MT | No | 7 |
| Ahmed 2014 (26) | Yes | No | NMT | MT | Yes | 6 |
| Chae 2015 (27) | Yes | No | NMT | MT | Yes | 8 |
| Hedelund 2012 (28) | Yes | No | NMT | MT | No | 5 |
| Faghihi 2016 (29) | Yes | No | NMT | MT | No | 8 |
| Politano 2019 (30) | Yes | No | NMT | MT | Yes | 7 |
MT, mentioned; NMT, not mentioned.
Effective efficiency of the CO2 lattice laser
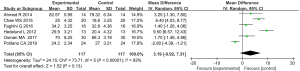
6 articles, containing evaluation of the effectiveness of treatment outcomes, met the requirements. For the effective rate of ultra-pulse CO2 fractional laser treatment (P<0.00001, I2=93%), the data were combined using the REM. The results showed that there was no statistically significant difference between the experimental group and the control group in terms of the effective rates for treating depressed acne scars [mean difference (MD) =3.19, 95% CI: –0.92 to 7.31; P=0.13; see Figure 4].
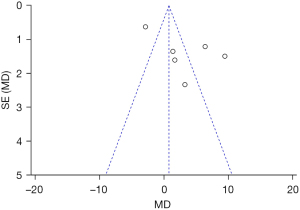
The effective rate of the ultra-pulse CO2 fractional laser treatment was taken as an indicator, and an inverted funnel chart as drawn. The results showed that the funnel chart was approximately symmetrical; thus, there was no obvious publication bias (see Figure 5).
Evaluation of skin depression
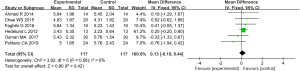
6 articles met the requirements and were included in this meta-analysis. For the skin depression score (P=0.58, I2=0%), the data of the articles were merged using a FEM. The results showed that there was no statistically significant difference in the skin depression scores between the 2 groups (MD =0.13, 95% CI: –0.18 to 0.44; P=0.42; see Figure 6).
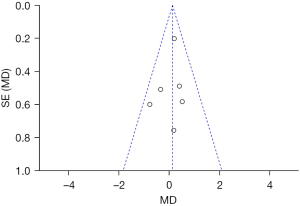
The skin depression score was taken as an indicator, and an inverted funnel chart was drawn. The results showed that the funnel chart was approximately symmetrical; thus, there was no obvious publication bias (see Figure 7).
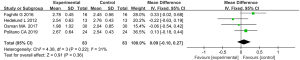
Evaluation of skin smoothness
4 articles which mentioned the skin smoothness evaluation met the requirements. In relation to the skin smoothness evaluation (P=0.45, I2=0%), the articles were homogeneous, and the FEM was used to merge the data. The results showed that the difference in the skin smoothness evaluations (SMD =0.49, 95% CI: 0.13–0.84; P=0.008) between the experimental group and the control group was statistically significant, such that after treatment, the skin smoothness of the experimental group was better than that of the control group (see Figure 8).
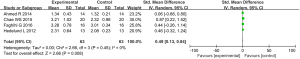
The skin smoothness evaluation was taken as an indicator, and an inverted funnel chart was drawn. The results showed that the funnel chart was approximately symmetrical; thus, there was no obvious publication bias (see Figure 9).
Evaluation on total score of skin lesions
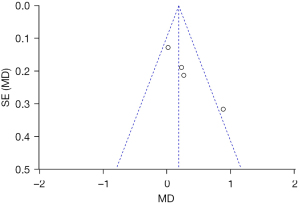
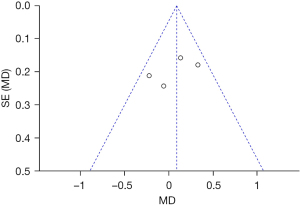
The total score of skin lesions was calculated as: depression degree score + smoothness score. A total of 4 articles reported on the total score of skin lesions. For the total score of skin lesions (P=0.87, I2=0%), the articles were homogeneous, and the FEM was used to merge the data. The results showed that the difference in the total scores of skin lesions (SMD =0.35, 95% CI: –0.00–0.70; P=0.05) between the experimental group and the control group was significantly different (see Figure 10).

The total score of skin lesions was taken as an indicator, and an inverted funnel chart was drawn. The results showed that the scattered points of the research object were slightly less symmetrical on the horizontal axis, suggesting that there may be publication bias (see Figure 11).
Evaluation on patient satisfaction
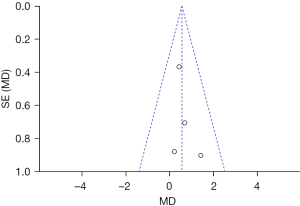
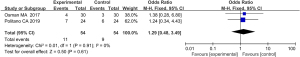
Patients were asked to evaluate their satisfaction with the treatment 2 months after the completion of the last treatment using a scale on which 3 = satisfied, 2 = very satisfied, 1 = generally satisfied, and 0 = dissatisfied. Four articles reported on patient satisfaction. For the evaluation of patient satisfaction (P=0.22, I2=31%), the indexes of each article were homogeneous, and the data were merged using a FEM. The results showed that there was no statistically significant difference between the experimental group and the control group in terms of patient satisfaction (MD =0.09, 95% CI: –0.10–0.27, P=0.36; see Figure 12).
Patient satisfaction was taken as an indicator, and an inverted funnel chart was drawn. The results showed that the distribution of the scattered points of the research object on the horizontal axis was slightly less symmetrical, indicating that there may be publication bias (see Figure 13).
Incidence of adverse reactions
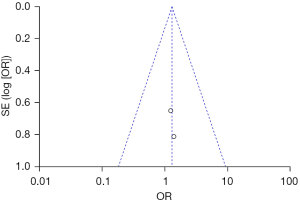
After treatment, possible adverse reactions in patients include pain, swelling, itching, hyperpigmentation, hypopigmentation, hyperplastic scars, skin infections, and herpes simplex. 2 articles reported on the incidence of post-treatment adverse reactions in patients. In relation to the incidence of adverse reactions (P=0.91, I2=0%), the articles were homogeneous, and the data were combined using a FEM. The results showed that the difference between the experimental group and the control group in relation to the incidence of adverse reactions (OR =1.29, 95% CI: 0.48–3.49, P=0.61) was not statistically significant (see Figure 14).
The incidence of adverse reactions was taken as an indicator, and an inverted funnel chart was drawn. The results showed that the scattered points of the study subjects were slightly less symmetrical on the horizontal axis, suggesting that there may be publication bias (see Figure 15).
Discussion
Ultra-pulse CO2 fractional laser treatments cause fibroblasts apoptosis, epidermis, and superficial dermis tissue vaporization and exfoliation through the photothermal action, and the initiation of the regeneration and repair program of the remaining stem cells to stimulate the growth of dermal collagen and the remodeling of the tissue structure to treat acne depressed scars (31). The imported lattice CO2 laser has deep and active modes. The deep mode uses a “low energy density, low coverage, small spot” treatment for the high-energy treatment of local skin lesions (32). The depth can reach the dermis layer to stimulate the proliferation of collagen, but its coverage rate is low, and the improvement of the superficial layer of the epidermis is poor. Conversely, the active mode uses “high energy density, high coverage, large spot” treatment to superficially grind the epidermis to promote epidermal renewal, and stimulates the growth factors secreted by keratinocytes to repair the thermal damage after the lasering (33). Studies have shown that after deep-mode treatment, the tissue produces vaporized micropillars extending 3–4 mm from the epidermis down to the reticular dermis layer, and a coagulation zone appears around each vaporized micropillar (34). The active mode produces extensive and superficial vaporization pits in the epidermis, and the depth can only reach the papillary dermis after increasing energy (35). Some scholars have used the lattice CO2 laser in deep mode combined with the active mode to treat acne depressed scars, and found an improvement rate of 37.6%±9.6% after 2 courses of treatment without any serious adverse reactions (36). In recent years, ultra-pulsed CO2 fractional laser combined with autologous platelet rich plasma (PRP) for the treatment of facial sunken acne scars has received certain attention. Ultra-pulsed CO2 fractional laser therapy has the effect of micro-creation, coupled with the characteristics of PRP to promote fast wound recovery, it can accelerate the reorganization and regeneration of dermal collagen after scar injury, and achieve desalination and regression of scars (37). At the same time, PRP may also inhibit the inflammatory response of the body by releasing antibacterial active peptides, releasing cytokines, etc., and play a role in improving pitted acne scars (38). The combination of the two uses the micro-creation effect of ultra-pulsed CO2 fractional laser and the mechanism of promoting wound healing by RPP. Scarred skin achieves collagen tissue remodeling during injury repair, and the dermal structure is rapidly formed to achieve or near complete re-epithelialization, thereby achieving regression and lightening of facial sunken acne scars (39). Current studies have pointed out that the ultra-pulsed CO2 fractional laser combined with PRP is more effective and safe than the ultra-pulsed CO2 fractional laser alone in the treatment of pitted acne scars (40).
This meta-analysis objectively evaluated the efficacy and safety of the ultra-pulse CO2 fractional laser in the treatment of depressed acne scars to provide a basis for its clinical application. The results of the study showed that ultra-pulse CO2 fractional laser treatment is more effective than other treatments, and patients receiving this type of treatment have higher levels of skin smoothness. The outcome indicators used in most studies are not uniform, and some articles have not provided data with standard deviations that can be used in meta-analyses. Indeed, most studies have only provided descriptive analyses of their research methods and results, which may be different. In addition, the size of samples in the included RCTs was small. It is hoped that more literature and research objects can be included in future meta-analyses. In recent years, with the development of science and the improvement of technical level, the photoelectric treatment of depressed acne scars has also developed rapidly, and new treatment modes and methods have also emerged. Fractional laser, as a minimally invasive treatment method, shows good application prospects in skin beauty (41). Although some basic research has been done, the treatment methods and energies reported in the literature can only be used as some references. In clinical practice, the treatment should be flexibly adjusted according to the actual situation of the patient's own conditions, equipment conditions, and doctor's experience. Some treatment theories and methods still lack multi-center, large-sample, randomized controlled clinical trials, and the related problems and long-term effects of their treatment still need further research.
In summary, the results of this study proved that for the treatment of depressed acne scars, the effective rates of ultra-pulse CO2 fractional laser treatment, and patients’ skin smoothness scores were better than those of other treatments. This study provides a basis for the clinical treatment of depressed acne scars with ultra-pulse CO2 fractional laser treatment, but more large-sample clinical trials on adverse reactions of ultra-pulsed CO2 fractional laser therapy, and more systematic meta-analyses need to be conducted to further evaluate the use of the ultra-pulse CO2 fractional laser in the treatment of depressed acne scars.
Conclusions
In this study, the therapeutic effects and adverse reactions related to the use of the ultra-pulse CO2 fractional laser in the treatment of depressed acne scars were analyzed via a meta-analysis. A total of 6 articles were included in the meta-analysis. The results showed that this treatment produced good effects in terms of its effectiveness rates and patient skin smoothness scores.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-70/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-70/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Knutsen-Larson S, Dawson AL, Dunnick CA, et al. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin 2012;30:99-106. [Crossref] [PubMed]
- Dreno B, Bagatin E, Blume-Peytavi U, et al. Female type of adult acne: Physiological and psychological considerations and management. J Dtsch Dermatol Ges 2018;16:1185-94. [Crossref] [PubMed]
- Clark AK, Haas KN, Sivamani RK. Edible Plants and Their Influence on the Gut Microbiome and Acne. Int J Mol Sci 2017;18:1070. [Crossref] [PubMed]
- Berry K, Lim J, Zaenglein AL. Acne Vulgaris: Treatment Made Easy for the Primary Care Physician. Pediatr Ann 2020;49:e109-15. [Crossref] [PubMed]
- Mahmood SN, Bowe WP. Diet and acne update: carbohydrates emerge as the main culprit. J Drugs Dermatol 2014;13:428-35. [PubMed]
- Kircik LH. Advances in the Understanding of the Pathogenesis of Inflammatory Acne. J Drugs Dermatol 2016;15:s7-10. [PubMed]
- Niemeier V, Kupfer J, Gieler U. Acne vulgaris--psychosomatic aspects. J Dtsch Dermatol Ges 2006;4:1027-36. [Crossref] [PubMed]
- Gollnick H, Abanmi AA, Al-Enezi M, et al. Managing acne in the Middle East: consensus recommendations. J Eur Acad Dermatol Venereol 2017;31:4-35. [Crossref] [PubMed]
- Tamulevičius T, Juodėnas M, Klinavičius T, et al. Dot-Matrix Hologram Rendering Algorithm and its Validation through Direct Laser Interference Patterning. Sci Rep 2018;8:14245. [Crossref] [PubMed]
- Neonakis IK, Spandidos DA. Detection of carbapenemase producers by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS). Eur J Clin Microbiol Infect Dis 2019;38:1795-801. [Crossref] [PubMed]
- Cong J, Yang J, Zhao B, et al. Fabricating subwavelength dot-matrix surface structures of molybdenum by transient correlated actions of two-color femtosecond laser beams. Opt Express 2015;23:5357-67. [Crossref] [PubMed]
- Min L. Effect of carbon dioxide laser (10600nm) on hydroxyproline in mouse skin. Pak J Pharm Sci 2019;32:1387-93. [PubMed]
- Gusev AI. Interfacing matrix-assisted laser desorption/ionization mass spectrometry with column and planar separations. Fresenius J Anal Chem 2000;366:691-700. [Crossref] [PubMed]
- Jin W, Li Z, Jin Z, Jin C. A novel technique for treating atrophic facial scars in Asians using ultra-pulse CO2 laser. J Cosmet Dermatol 2020;19:1099-104. [Crossref] [PubMed]
- Wei LIU, Feng CHEN, Xiao-lei TAN. Efficacy and safety of ultra pulse CO2 fractional laser combined with recombinant bovine epidermal growth factor gel in the treatment of facial depressed scar after surgery. Journal of Hebei Medical University 2021;42:201.
- Lei Y, Wang Q, Tan J. Histological changes of the dermal-epidermal junction of superficial scarin rabbit ears induced by ultrapulse CO2 fractional laser. Chinese Journal of Medical Aesthetics and Cosmetology 2017;23:125-8.
- Omi T, Numano K. The Role of the CO2 Laser and Fractional CO2 Laser in Dermatology. Laser Ther 2014;23:49-60. [Crossref] [PubMed]
- Rodriguez-Menocal L, Davis SS, Becerra S, et al. Assessment of Ablative Fractional CO2 Laser and Er:YAG Laser to Treat Hypertrophic Scars in a Red Duroc Pig Model. J Burn Care Res 2018;39:954-62. [Crossref] [PubMed]
- Qu Y, Ma WY, Sun Q. The comparison of the rejuvenation effects on the skin of Wistar rats between 10600 nm CO2 fractional laser and retinoic acid. Eur Rev Med Pharmacol Sci 2017;21:1952-8. [PubMed]
- Ha L, Jaspan M, Welford D, et al. First Assessment of a Carbon Monoxide Laser and a Thulium Fiber Laser for Fractional Ablation of Skin. Lasers Surg Med 2020;52:788-98. [Crossref] [PubMed]
- Elsaie ML, Ibrahim SM, Saudi W. Ablative Fractional 10 600 nm Carbon Dioxide Laser Versus Non-ablative Fractional 1540 nm Erbium-Glass Laser in Egyptian Post-acne Scar patients. J Lasers Med Sci 2018;9:32-5. [Crossref] [PubMed]
- Ansari F, Sadeghi-Ghyassi F, Yaaghoobian B. The clinical effectiveness and cost-effectiveness of fractional CO2 laser in acne scars and skin rejuvenation: A meta-analysis and economic evaluation. J Cosmet Laser Ther 2018;20:248-51. [Crossref] [PubMed]
- Wan W, Huang W, Pu D, et al. High performance organic distributed Bragg reflector lasers fabricated by dot matrix holography. Opt Express 2015;23:31926-35. [Crossref] [PubMed]
- Flaxel C, Bradle J, Acott T, et al. Retinal pigment epithelium produces matrix metalloproteinases after laser treatment. Retina 2007;27:629-34. [Crossref] [PubMed]
- Osman MA, Shokeir HA, Fawzy MM. Fractional Erbium-Doped Yttrium Aluminum Garnet Laser Versus Microneedling in Treatment of Atrophic Acne Scars: A Randomized Split-Face Clinical Study. Dermatol Surg 2017;43:S47-56. [Crossref] [PubMed]
- Ahmed R, Mohammed G, Ismail N, et al. Randomized clinical trial of CO2 LASER pinpoint irradiation technique versus chemical reconstruction of skin scars (CROSS) in treating ice pick acne scars. J Cosmet Laser Ther 2014;16:8-13. [Crossref] [PubMed]
- Chae WS, Seong JY, Jung HN, et al. Comparative study on efficacy and safety of 1550 nm Er:Glass fractional laser and fractional radiofrequency microneedle device for facial atrophic acne scar. J Cosmet Dermatol 2015;14:100-6. [Crossref] [PubMed]
- Hedelund L, Haak CS, Togsverd-Bo K, et al. Fractional CO2 laser resurfacing for atrophic acne scars: a randomized controlled trial with blinded response evaluation. Lasers Surg Med 2012;44:447-52. [Crossref] [PubMed]
- Faghihi G, Keyvan S, Asilian A, et al. Efficacy of autologous platelet-rich plasma combined with fractional ablative carbon dioxide resurfacing laser in treatment of facial atrophic acne scars: A split-face randomized clinical trial. Indian J Dermatol Venereol Leprol 2016;82:162-8. [Crossref] [PubMed]
- Politano CA, Costa-Paiva L, Aguiar LB, et al. Fractional CO2 laser versus promestriene and lubricant in genitourinary syndrome of menopause: a randomized clinical trial. Menopause 2019;26:833-40. [Crossref] [PubMed]
- Wang H, Guo B, Hui Q, et al. CO2 lattice laser reverses skin aging caused by UVB. Aging (Albany NY) 2020;12:7056-65. [Crossref] [PubMed]
- Zhang L, Lai Y, Pan W, et al. Application of ultra pulse CO2 lattice laser in the treatment of female urinary incontinence. Transl Androl Urol 2021;10:2471-7. [Crossref] [PubMed]
- Yoshida M, De Zoysa M, Ishizaki K, et al. Double-lattice photonic-crystal resonators enabling high-brightness semiconductor lasers with symmetric narrow-divergence beams. Nat Mater 2019;18:121-8. [Crossref] [PubMed]
- Ho G, Barbenel J, Grant MH. Effect of low-level laser treatment of tissue-engineered skin substitutes: contraction of collagen lattices. J Biomed Opt 2009;14:034002. [Crossref] [PubMed]
- Li H, Ki H. Lattice-Boltzmann simulation of laser interaction with weakly ionized helium plasmas. Phys Rev E Stat Nonlin Soft Matter Phys 2010;82:016703. [Crossref] [PubMed]
- Ates C, Pohl T, Pattard T, et al. Antiblockade in Rydberg excitation of an ultracold lattice gas. Phys Rev Lett 2007;98:023002. [Crossref] [PubMed]
- Wu N, Sun H, Sun Q, et al. A meta-analysis of fractional CO2 laser combined with PRP in the treatment of acne scar. Lasers Med Sci 2021;36:1-12. [Crossref] [PubMed]
- Chang HC, Sung CW, Lin MH. Efficacy of Autologous Platelet-Rich Plasma Combined With Ablative Fractional Carbon Dioxide Laser for Acne Scars: A Systematic Review and Meta-Analysis. Aesthet Surg J 2019;39:NP279-NP287. [Crossref] [PubMed]
- Abdel Aal AM, Ibrahim IM, Sami NA, Abdel Kareem IM. Evaluation of autologous platelet-rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther 2018;20:106-113. [Crossref] [PubMed]
- Mu YZ, Jiang L, Yang H. The efficacy of fractional ablative carbon dioxide laser combined with other therapies in acne scars. Dermatol Ther 2019;32:e13084. [PubMed]
- Dai Z, Lou X, Shen T, et al. Combination of ablative fractional carbon dioxide laser and platelet-rich plasma treatment to improve hypertrophic scars: a retrospective clinical observational study. Burns Trauma 2021;9:tkab016.





