
Association among sleep, depression, and health-related quality of life in patients with non-dialysis chronic kidney disease during the coronavirus disease 2019 pandemic
Introduction
Sleep disturbance, mainly including insomnia, sleep apnea and restless legs syndrome, has attracted wide attention in recent years for its significant impact on individual health. A large multicenter study showed that the prevalence of sleep disturbances in dialysis patients was approximately 49% and was associated independently with heightened psychological distress, poorer health-related quality of life (HRQOL), and higher mortality (1). More recently, the reported prevalence ranged from 20% to 70% worldwide (2,3). Other researches have also demonstrated an association between sleep disorders and aggravating burden of kidney disease, higher probability of depression, and impaired HRQOL in patients receiving dialysis (4-9).
In contrast to the numerous published researches on the relationship between sleep disturbance, depression, and HRQOL in patients on dialysis, few published researches have studied whether sleep affects these health outcomes in patients with non-dialysis chronic kidney disease (CKD). Several recent studies reported that inappropriate sleep duration was independently associated with low HRQOL in adults with CKD. But they only focused on sleep duration, which is just one aspect of sleep, cannot represent the overall sleep situation (10,11).
Consequently, the prevalence of sleep disturbance and its impacts on patient wellbeing in non-dialysis CKD patients still need to be explored, especially during the coronavirus disease 2019 (COVID-19) epidemic, a global health crisis that can damage both physical health and mental health (12). The aims of this study are to (I) estimate the prevalence of sleep disturbance during the COVID-19 pandemic; (II) investigate associations between sleep, HRQOL, and depressive symptoms.
We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3416/rc).
Methods
Patients and settings
Convenience sampling was adopted in the present study. 172 Chinese adults with CKD not on dialysis were recruited to the cross-sectional survey from Tangdu hospital between June 1, 2020 and August 31, 2021. In this study, we defined CKD as eGFR<60 mL/min per 1.73 m2 or proteinuria ≥2+ (>300 mg/dL) or structural abnormalities for >3 months. eGFR was calculated based on the CKD Epidemiology Collaboration equation (13). Participants ranged in age from 18 to 75. We excluded subjects with maintenance dialysis, history of malignancy, advanced heart failure, liver cirrhosis, psychotic symptoms, and have difficulty in hearing or reading the questionnaires. Upon enrollment, all subjects were asked to accomplish a questionnaire, which collected data on self-reported health measures employing verified instruments regarding sleep, HRQOL, and depressive symptoms, as well as some baseline characteristics including demographic data, comorbid conditions, and medications. Laboratory values were extracted from patient records. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Tangdu Hospital (No. TDLL-202201-01) and informed consent was taken from all the patients.
Cardiovascular disease (CVD) history, diabetes, hypertension, hyperuricemia, hyperlipidemia, and anemia were recorded in detail. History of CVD was defined as angina pectoris, cerebrovascular accident, myocardial infarction, transient ischemic attack, revascularization, or peripheral artery disease, diagnosed in the medical history. Diabetes was defined as a fasting glucose level ≥7.0 mmol/L, glycated hemoglobin ≥6.5% or use of antidiabetic drugs. Hypertension was defined as diastolic blood pressure ≥90 mmHg or systolic blood pressure ≥140 mmHg or taking antihypertensive medication. Hyperuricemia was defined as serum uric acid greater than 6.6 mg/dL in women or 7.7 mg/dL in men. Hyperlipidemia was defined as low-density lipoprotein cholesterol ≥160 mg/dL according to the Chinese Guidelines for the Management of Dyslipidemia in Adults. Anemia is defined as blood hemoglobin <120 g/L or hematocrit <37% in adult females and Hb concentration <130 g/L or Hct <39% in adult males. “glucocorticoid use” refers to the use of any type of glucocorticoid in the last year, either orally or intravenously.
Assessment of sleep
Sleep was assessed with the Chinese version (14) of the Pittsburgh Sleep Quality Index (PSQI) (15). The questionnaire comprises 19 items, relates to the last 1-month time period, and generates seven component scores, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The seven component scores are summed to give one global score (range, 0–21). Poor sleep was defined as PSQI global score >5, a widely accepted cutoff value (16). Previous researches have demonstrated the Chinese version of the PSQI has good internal consistency (Cronbach’s a =0.82–0.83) and test-retest reliability (r =0.77–0.85) (14).
Assessment of HRQOL
HRQOL was measured by the Kidney Disease Quality of Life 36-Item Short-Form Survey (KDQOL-36), which includes the 12-item Short-Form Health Survey (SF-12) as its generic core and 3 kidney disease-targeted scales: burden of kidney disease, symptoms and problems of kidney disease, and effects of kidney disease (17). SF-12 scores are summarized as physical component summary (PCS) and mental component summary (MCS). Responses to each question were transformed into scores ranging from 0 to 100 using an excel scoring template. The higher the score, the greater the HRQOL. Dialysis patients were not included in our study, so dialysis-related issues were omitted by KDQOL-36.
Assessment of depression
All participants were asked to complete the 9-item Patient Health Questionnaire (PHQ-9), a 9-item tool that identifies probable cases of depression and to assess symptom severity over the prior 2 weeks. The PHQ-9 has been validated as a measure of depressive disorders for patients with CKD (18,19). Nine questions are on a scale from 0 (“not at all”) to 3 (“nearly every day”), providing a PHQ-9 range from 0 to 27. PHQ-9 score ≥5 is considered having depressive symptoms.
Statistical analysis
Tests of normality were performed by K-S test and Q-Q plot. Cross-sectional data of participants’ characteristics (mean ± SD, median [interquartile range], or proportion) were calculated according to the presence or absence of sleep disorders. Differences between good sleepers and poor sleepers groups in demographic characteristics, clinical variables, depression, and HRQOL were compared using t-test, Mann-Whitney U-test, Kruskal-Wallis test for continuous variables, and the χ2 statistic for categorical variables. Linear regression analysis was used to assess the influence of sleep on group differences in depression and HRQOL. Candidate variables with significant test statistics (P<0.2) in univariate analysis were selected for the multivariable model. To test the mediation effects of depression between sleep and HRQOL, we employed the bootstrap method. A two-sided P<0.05 was considered statistically significant. All analyzes were performed using SPSS 20 software.
Results
Prevalence of sleep disturbance and related patient characteristics
Table 1 shows sociodemographic and clinical characteristics of participants divided by good and poor sleepers. Of the 172 patients, 100 (58%) met the criteria for poor sleepers by the PSQI. Except for age, anemia, and glucocorticoid use, there were no statistically significant inter-group differences. The descriptive statistics of PSQI components in different CKD stages was presented in Table S1. For the component “use of sleep medication”, only several patients in G1 had taken sleep medications, while all patients in G2–5 were not involved in that. In addition to this, all other components were problematic in CKD patients. There were statistical differences in sleep duration among patients of G1–5. However, no significant difference was found in the profile of other six components.
Table 1
| Overall (N=172) | Good sleepers (N =72) | Poor sleepers (N=100) | P value | |
|---|---|---|---|---|
| Age | 51.00 (38.00–60.75) | 48.00 (37.00–59.50) | 52.00 (40.00–61.00) | 0.43 |
| Age groups | 0.01 | |||
| ≤45 y | 63 (36.6%) | 32 (44.4%) | 31 (31.0%) | |
| 46–65 y | 85 (49.4%) | 26 (36.1%) | 59 (59.0%) | |
| >65 y | 24 (14.0%) | 14 (19.5%) | 10 (10.0%) | |
| Gender | 0.22 | |||
| Male | 86 (50.0%) | 40 (55.6%) | 46 (46.0%) | |
| Female | 86 (50.0%) | 32 (44.4%) | 54 (54.0%) | |
| BMI | 0.83 | |||
| <28 | 130 (75.6%) | 55 (76.4%) | 75 (75.0%) | |
| ≥28 | 42 (24.4%) | 17 (23.6%) | 25 (25.0%) | |
| Marital status | 0.69 | |||
| Married | 157 (91.3%) | 65 (90.3%) | 92 (92.0%) | |
| Others | 15 (8.7%) | 7 (9.7%) | 8 (8.0%) | |
| Employment status | 0.78 | |||
| Nonprofessional | 115 (66.9%) | 49 (68.1%) | 66 (66.0%) | |
| Professional | 57 (33.1%) | 23 (31.9%) | 34 (34.0%) | |
| Place of residence | 0.79 | |||
| Urban | 119 (69.2%) | 49 (68.1%) | 70 (70.0%) | |
| Rural | 53 (30.8%) | 23 (31.9%) | 30 (30.0%) | |
| Education | 0.71 | |||
| Below high school | 76 (44.2%) | 33 (45.8%) | 43 (43.0%) | |
| High school and above | 96 (55.8%) | 39 (54.2%) | 57 (57.0%) | |
| Monthly income | 0.80 | |||
| <¥1,000 | 38 (22.1%) | 18 (25.0%) | 20 (20.0%) | |
| ¥1,000–5,000 | 81 (47.1%) | 34 (47.2%) | 47 (47.0%) | |
| ¥5,000–10,000 | 38 (22.1%) | 15 (20.8%) | 23 (23.0%) | |
| >¥10,000 | 15 (8.7%) | 5 (7.0%) | 10 (10.0%) | |
| CKD diagnosis time | 0.85 | |||
| <1 y | 77 (44.8%) | 30 (41.7%) | 47 (47.0%) | |
| 1–5 y | 52 (30.2%) | 24 (33.3%) | 28 (28.0%) | |
| 5–10 y | 30 (17.4%) | 12 (16.7%) | 18 (18.0%) | |
| >10 y | 13 (7.6%) | 6 (8.3%) | 7 (7.0%) | |
| Comorbid condition | ||||
| Cardiovascular disease | 19 (11.0%) | 5 (6.9%) | 14 (14.0%) | 0.15 |
| Hypertension | 93 (54.1%) | 37 (51.4%) | 56 (56.0%) | 0.55 |
| Diabetes | 38 (22.1%) | 15 (20.8%) | 23 (23.0%) | 0.74 |
| Hyperuricemia | 66 (38.4%) | 28 (38.9%) | 38 (38.0%) | 0.91 |
| Hyperlipidemia | 80 (46.5%) | 30 (41.7%) | 50 (50.0%) | 0.28 |
| Anemia | 55 (32.0%) | 17 (23.6%) | 38 (38.0%) | 0.04 |
| Glucocorticoid use | 95 (55.2%) | 48 (66.7%) | 47 (47.0%) | 0.01 |
| Tobacco use | 35 (20.3%) | 18 (25.0%) | 17 (17.0%) | 0.20 |
| Biochemical Indicators | ||||
| Serum creatinine | 75.45 [51.60–141.65] | 75.60 [52.08–131.78] | 75.45 [51.15–148.50] | 0.88 |
| Blood urea nitrogen | 6.85 [5.13–11.45] | 7.00 [5.23–10.80] | 6.80 [5.01–11.58] | 0.82 |
| Blood uric acid | 349.17±107.10 | 355.54±121.37 | 344.59±95.90 | 0.51 |
| Serum albumin | 38.50 [30.23–43.60] | 40.05 [30.90–43.48] | 38.25 [29.98–43.90] | 0.69 |
| Albumin globulin ratio | 1.79±0.49 | 1.86±0.54 | 1.75±0.45 | 0.15 |
| eGFR | 91.14 [42.28–110.31] | 91.14 [45.61–109.83] | 88.92 [38.64–111.03] | 0.73 |
| eGFR stages | 0.64 | |||
| <30 | 115 (66.9%) | 51 (70.8%) | 64 (64.0%) | |
| 30–60 | 21 (12.2%) | 8 (11.1%) | 13 (13.0%) | |
| >60 | 36 (20.9%) | 13 (18.1%) | 23 (23.0%) |
Results are expressed as proportion, median [interquartile range] or mean ± SD. P values were derived from t-test, Mann-Whitney U-test, or χ2 statistic as appropriate. BMI, body mass index; eGFR, estimated glomerular filtration rate; y, year; ¥, yuan.
HRQOL and depression scores according to sleep
The scores of five dimensions of KDQOL-36, as well as global score and conclusions of PHQ-9 in the good sleepers vs. poor sleepers groups, respectively, are demonstrated in Table 2. Compared to patients with good sleep, those with poor sleep had statistically and clinically significantly lower scores (defined by a reduction >5 points) for the symptoms, effect, and burden of kidney disease. They also reported lower PCS (36.80 vs. 41.96; P<0.05) and MCS (41.96 vs. 54.46; P<0.05). The most significant difference was found in the area known as the burden of kidney disease numerically. In terms of depression, the median PHQ-9 score in the poor sleepers was 7 compared to 2 in the good sleepers (P<0.001), indicating a higher possibility of depression in the population with poor sleep. Proportionately, 73.0% of poor sleepers exhibited different levels of depressive symptoms, but just 16.7% of good sleepers.
Table 2
| Overall (N=172) | Good sleepers (N=72) | Poor sleepers (N=100) | P value | |
|---|---|---|---|---|
| HRQOL | ||||
| Symptoms and problems | 88.64 [75.00–95.45] | 95.45 [88.64–97.73] | 81.25 [70.45–90.91] | <0.001 |
| Effects of kidney disease | 78.13 [62.50–90.63] | 84.38 [68.75–90.63] | 75.00 [57.03–87.50] | 0.002 |
| Burden of kidney disease | 43.75 [25.00–79.69] | 65.63 [37.50–87.50] | 37.50 [8.75–60.94] | <0.001 |
| PCS | 39.34 [32.77–47.93] | 41.96 [36.04–49.89] | 36.80 [31.21–45.45] | 0.001 |
| MCS | 52.85 [43.61–57.38] | 54.46 [45.84–58.53] | 50.91 [39.68–56.67] | 0.005 |
| Depression | ||||
| PHQ-9 score | 4 [2–8] | 2 [1–4] | 7 [4–10] | <0.001 |
| PHQ-9 score ≥5 | <0.001 | |||
| No | 87 (50.6%) | 60 (83.3%) | 27 (27.0%) | |
| Yes | 85 (49.4%) | 12 (16.7%) | 73 (73.0%) |
Values are median [interquartile range] or proportion. Higher scores indicate increased HRQOL and depression. P values were derived from Mann-Whitney U-test and χ2 statistic. HRQOL, health-related quality of life; PCS, physical component summary; MCS, mental component summary; PHQ-9, 9-item Patient Health Questionnaire.
Associations of sleep disturbance with HRQOL and depressive symptoms
To further evaluate if sleep disturbance is associated with the various HRQOL dimensions and depression independent of clinical characteristics and socio-demographic, univariate and multivariate models were established, as shown in Table 3. In unadjusted models, sleep was related to changes of HRQOL and depression scores statistically significant. After controlling for screened elements, the association of sleep disturbance with reduced HRQOL and increased depression in each area remained remarkable in the adjusted models. We have also provided the correlation coefficients between PSQI and KDQOL-36 domains, see in Table S2.
Table 3
| Unadjusted coefficient | Adjusted coefficient | ||||
|---|---|---|---|---|---|
| B (95% CI) | P value | B (95% CI) | P value | ||
| PCS | –0.26 (–0.41 to –0.11) | 0.001 | –0.26 (–0.41 to –0.12) | <0.001a | |
| MCS | –0.23 (–0.38 to –0.08) | 0.002 | –0.22 (–0.37 to –0.08) | 0.003b | |
| Symptoms and problems | –0.44 (–0.57 to –0.30) | <0.001 | –0.44 (–0.58 to –0.30) | <0.001c | |
| Effects of kidney disease | –0.25 (–0.40 to –0.11) | 0.001 | –0.27 (–0.41 to –0.12) | <0.001d | |
| Burden of kidney disease | –0.31 (–0.46 to –0.17) | <0.001 | –0.34 (–0.48 to –0.20) | <0.001e | |
| PHQ-9 scores | 0.55 (0.43 to 0.68) | <0.001 | 0.53 (0.41 to 0.66) | <0.001f | |
Univariate and multivariate linear regression models were employed in analyses, and multivariate model included variables significantly associated with the above areas in the univariate analysis. a, the model is adjusted for place of residence, education, monthly income, CKD diagnosis time and anemia; b, the model is adjusted for age, place of residence, diabetes, and hyperuricemia; c, the model is adjusted for education, monthly income, diabetes, anemia, hormone use and eGFR; d, the model is adjusted for monthly income; e, the model is adjusted for place of residence, education, employment status, monthly income, anemia, hyperuricemia, hyperlipidemia, hormone use and eGFR. f, the model is adjusted for hyperuricemia and hormone use. HRQOL, health-related quality of life; CKD, chronic kidney disease; CI, confidence interval; PCS, physical component summary; MCS, mental component summary; PHQ-9, 9-item Patient Health Questionnaire.
Depression mediates the link between sleep and HRQOL
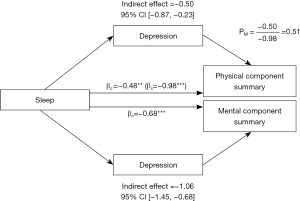
To investigate the relationship between sleep disorders, depression, and HRQOL, we hypothesized and tested the mediating role of depression. We employed bootstrapping mediation analysis, which demonstrated that depression is a significant mediator (β=‒0.50, 95% CI: ‒0.87 to ‒0.23) explaining 51% (PM=0.51) of the relationship between sleep and PCS. However, the direct effect of sleep on MCS is not significant (P=0.10), so depression played a fully mediating role in the association between sleep disorders and MCS. The specific relationships and data are represented in Figure 1.
Discussion
In this cross-sectional study of patients with non-dialysis CKD, we verified important associations of self-reported sleep with depression as well as HRQOL and had some major discoveries. First, the overall prevalence of sleep disturbance was 58%, meaning that more than half of the non-dialysis CKD population suffered from poor sleep during the COVID-19 pandemic. Second, sleep disorders were associated with increasingly poor HRQOL and depressive symptoms. Third, depression mediated the effects of sleep disturbance on the PCS and MCS of HRQOL in non-dialysis CKD patients.
In prior reports of CKD patients not on dialysis, the overall prevalence of sleep disturbances was between 20‒40% (20-22). The proportion of PSQI global score >5 in our study was higher than the rates, suggesting a significant increase in the incidence of sleep disorders during the COVID-19 pandemic. That is, the COVID-19 pandemic had a negative effect on the sleep of non-dialysis CKD, which was in accord with studies of other populations (23).
In our study, distinctions in sleep among patients with non-dialysis CKD were explained largely by age, anemia, and glucocorticoid use, similar to the results reported in previous studies (19,24-26). A large-scale study found that time-in-bed and total sleep time showed a U-shaped association with age, which further corroborated the reliability of our results (27). Anemia, a common complication of CKD, contributes to sleep issues frequently (28). This could be explained by several factors: iron deficiency impairs certain neurotransmitters needed for sleep, such as norepinephrine, serotonin, and dopamine. In the meantime, anemia might disrupt sleep by causing restless legs syndrome, a symptom widely present in CKD patients (29). Glucocorticoids, the final mediators of the hypothalamic-pituitary-adrenal axis cascade, are critical to the pathogenesis of sustained stress-related sleep disorders (30). But our data indicated the use of glucocorticoids was beneficial for sleep, possibly because glucocorticoids ease the pain of the disease, leading to improved sleep. Meanwhile, different to several studies (19,31) which have reported that females and those with lower eGFR in dialysis patients are more susceptible to poor sleep, our results suggested that there were no differences in gender or eGFR between good sleepers and poor sleepers. The most likely reason for this is the difference in the subjects studied. Several additional factors we didn’t consider may also influence sleep situations. According to the reports, there is a complex interaction between inflammatory markers and sleep. Some inflammatory cytokines could affect sleep-wake regulation (32-34), while sleep deprivation increases inflammatory reactivity (35-37). The regression model adjusted for critical factors in a cross-sectional study showed that constipation-related symptoms were independently associated with the incidence of sleep disorders (38). Moreover, different treatments and side effects of medications such as calcium channel blockers have impacts on sleep (39,40). Patients with sleep disturbance had over four times the prevalence of depression and a global reduction in HRQOL when compared with those without sleep disorders, indicating increased distress of poor sleepers. The results of multiple linear regression models revealed that the psychological status and HRQOL of good sleepers remained better even after adjustment for demographic and clinical variables. The strong correlation between sleep disturbance and depression, as well as HRQOL in patients with CKD not on dialysis, paralleled prior discoveries of the link seen between impaired sleep quality and these self-reported outcomes in dialysis patients.
Previous researches have demonstrated that there is a high comorbidity between sleep disturbance and depression (41-43). Recently, the directional relationship of the two has been extensively explored. A longitudinal study reported by Breslau et al. supported that sleep disorders were likely to precede depression, not the other way around (44). Meta-analyses have suggested poor sleep is recognized as a frequent precursor to the development of depression (45). Many other studies have also proved this view by building models such as the structural equation model and cross-lagged panel model (46,47). Meanwhile, the strong correlation between depression and HRQOL led to depression often playing a mediating role in the relationship between certain factors and HRQOL. For example, depression could mediate the impact of clinical pain on HRQOL in fibromyalgia (48), as well as the effect of seizure frequency on HRQOL in patients with epilepsy (49). And most importantly, psychological disturbance played a mediating role in the relationship between sleep disturbance and HRQOL in patients on dialysis (50). Based on the above studies and what we’ve proven about the relationship between the three, we hypothesized and examined whether depression mediated the association between sleep status and HRQOL in patients with non-dialysis CKD. The results suggest that depression significantly mediates the relationship between sleep and HRQOL, which may be explained by a working mechanism of a physio-psycho-physiological cycle. Depression could be a psychological trigger for HRQOL and sleep is a significant factor in tipping the trigger. Sleep disorders and depression share a bidirectional relationship that reinforces each other, which ultimately lead to a decline in HRQOL (51,52). These conclusions underscore the importance of symptom management on HRQOL. Future studies of sleep and psychological interventions among people with non-dialysis CKD should be a priority.
Several limitations are worth noting. First, the generalizability of the results might be weakened due to the relatively small sample size. Second, polysomnography and actigraphy, which monitor sleep situations objectively, were not employed in this research. Instead, we relied solely on patients’ self-reports. Third, depressive symptoms and sleep disorders were defined as self-reported questionnaire scores above selected cut-offs, therefore we can’t make a clinical diagnosis. Fourth, some information related to COVID-19 outbreak was not assessed because of the lack of standardized questionnaires, such as whether they had difficulty in hospitalizing for the COVID-19 pandemic restrictions. Finally, the cross-sectional analysis of the study made it impossible to draw causal conclusions between sleep disturbances and other variables (23).
In conclusion, our data showed the high prevalence of sleep disturbances as well as the strong associations between sleep disorders, depressive symptoms, and HRQOL in patients with non-dialysis CKD during the COVID-19 pandemic. Therapy of the underlying diseases has only partial effects on HRQOL in patients with CKD, therefore, attention should be paid to treatable health-related domains such as sleep and depression. Future prospective, interventional studies may assess the potential contribution of drugs to sleep and psychology, as well as the benefits of relevant treatment regimens on patient health outcomes. Nephrologists should be aware of the severity of sleep disturbance and depression in patients with non-dialysis CKD and consider screening patients for poor sleep and mental health to find out who might benefit from treatments that may provide relief (53,54), with the ultimate objective to improve the HRQOL, especially during the COVID-19 pandemic.
Acknowledgments
Funding: This work was supported by grants from Natural Science Basic Research Plan in Shaanxi Province of China (No. 2019JM-033); Technology Innovation Development Foundation of Tangdu Hospital (Nos. 2019QYTS003, 2020XKPT014 and 2021QYJC-001); and Nursing Research Foundation of Tangdu Hospital (No. TDHLKY-2019-05).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3416/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3416/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3416/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elder SJ, Pisoni RL, Akizawa T, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2008;23:998-1004. [Crossref] [PubMed]
- Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int 2015;88:447-59. [Crossref] [PubMed]
- Lindner AV, Novak M, Bohra M, et al. Insomnia in Patients With Chronic Kidney Disease. Semin Nephrol 2015;35:359-72. [Crossref] [PubMed]
- De Silva I, Evangelidis N, Hanson CS, et al. Patient and caregiver perspectives on sleep in dialysis. J Sleep Res 2021;30:e13221. [Crossref] [PubMed]
- Kutner NG, Zhang R, Huang Y, et al. Association of sleep difficulty with Kidney Disease Quality of Life cognitive function score reported by patients who recently started dialysis. Clin J Am Soc Nephrol 2007;2:284-9. [Crossref] [PubMed]
- Unruh ML, Buysse DJ, Dew MA, et al. Sleep quality and its correlates in the first year of dialysis. Clin J Am Soc Nephrol 2006;1:802-10. [Crossref] [PubMed]
- Iliescu EA, Coo H, McMurray MH, et al. Quality of sleep and health-related quality of life in haemodialysis patients. Nephrol Dial Transplant 2003;18:126-32. [Crossref] [PubMed]
- Zheng C, Xu J, Chen C, et al. Effects of sleep disorders and sedative-hypnotic medications on health-related quality of life in dialysis patients. Int Urol Nephrol 2019;51:163-74. [Crossref] [PubMed]
- Intas G, Rokana V, Stergiannis P, et al. Sleeping Disorders and Health-Related Quality of Life in Hemodialysis Patients with Chronic Renal Disease in Greece. Adv Exp Med Biol 2020;1196:73-83. [Crossref] [PubMed]
- Sung SA, Hyun YY, Lee KB, et al. Sleep Duration and Health-Related Quality of Life in Predialysis CKD. Clin J Am Soc Nephrol 2018;13:858-65. [Crossref] [PubMed]
- Lee HJ, Kwak N, Kim YC, et al. Impact of Sleep Duration on Mortality and Quality of Life in Chronic Kidney Disease: Results from the 2007-2015 KNHANES. Am J Nephrol 2021;52:396-403. [Crossref] [PubMed]
- Creese B, Khan Z, Henley W, et al. Loneliness, physical activity, and mental health during COVID-19: a longitudinal analysis of depression and anxiety in adults over the age of 50 between 2015 and 2020. Int Psychogeriatr 2021;33:505-14. [Crossref] [PubMed]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. [Crossref] [PubMed]
- Tsai PS, Wang SY, Wang MY, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res 2005;14:1943-52. [Crossref] [PubMed]
- Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193-213. [Crossref] [PubMed]
- Mollayeva T, Thurairajah P, Burton K, et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev 2016;25:52-73. [Crossref] [PubMed]
- Hays RD, Kallich JD, Mapes DL, et al. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 1994;3:329-38. [Crossref] [PubMed]
- Han Y, Song X, Liu Y, et al. The effects of depression and age on sleep disturbances in patients with non-dialysis stage 3-5 chronic kidney disease: a single-center study. Int Urol Nephrol 2020;52:739-48. [Crossref] [PubMed]
- Pai MF, Hsu SP, Yang SY, et al. Sleep disturbance in chronic hemodialysis patients: the impact of depression and anemia. Ren Fail 2007;29:673-7. [Crossref] [PubMed]
- Tu CY, Chou YH, Lin YH, et al. Sleep and emotional disturbance in patients with non-dialysis chronic kidney disease. J Formos Med Assoc 2019;118:986-94. [Crossref] [PubMed]
- Huang Z, Tang X, Zhang T, et al. Prevalence of sleep apnoea in non-dialysis chronic kidney disease patients: A systematic review and meta-analysis. Nephrology (Carlton) 2019;24:1041-9. [Crossref] [PubMed]
- Lee J, Nicholl DD, Ahmed SB, et al. The prevalence of restless legs syndrome across the full spectrum of kidney disease. J Clin Sleep Med 2013;9:455-9. [Crossref] [PubMed]
- Terán-Pérez G, Portillo-Vásquez A, Arana-Lechuga Y, et al. Sleep and Mental Health Disturbances Due to Social Isolation during the COVID-19 Pandemic in Mexico. Int J Environ Res Public Health 2021;18:2804. [Crossref] [PubMed]
- Ezzat H, Mohab A. Prevalence of sleep disorders among ESRD patients. Ren Fail 2015;37:1013-9. [Crossref] [PubMed]
- Kusleikaite N, Bumblyte IA, Razukeviciene L, et al. Sleep disorders and quality of life in patients on hemodialysis. Medicina (Kaunas) 2005;41:69-74. [PubMed]
- Bae H, Kim KT, Heo MH, et al. The prevalence and clinical characteristics of restless legs syndrome in patients with iron deficiency anemia in Korea. J Clin Sleep Med 2021;17:1447-52. [Crossref] [PubMed]
- Li L, Nakamura T, Hayano J, et al. Age and gender differences in objective sleep properties using large-scale body acceleration data in a Japanese population. Sci Rep 2021;11:9970. [Crossref] [PubMed]
- Hansen RA, Chin H, Blalock S, et al. Predialysis chronic kidney disease: evaluation of quality of life in clinic patients receiving comprehensive anemia care. Res Social Adm Pharm 2009;5:143-53. [Crossref] [PubMed]
- Kutner NG, Zhang R, Huang Y, et al. Racial differences in restless legs symptoms and serum ferritin in an incident dialysis patient cohort. Int Urol Nephrol 2012;44:1825-31. [Crossref] [PubMed]
- Wang ZJ, Zhang XQ, Cui XY, et al. Glucocorticoid receptors in the locus coeruleus mediate sleep disorders caused by repeated corticosterone treatment. Sci Rep 2015;5:9442. [Crossref] [PubMed]
- Petrov ME, Kim Y, Lauderdale DS, et al. Objective sleep, a novel risk factor for alterations in kidney function: the CARDIA study. Sleep Med 2014;15:1140-6. [Crossref] [PubMed]
- Abegunde AT, Muhammad BH, Bhatti O, et al. Environmental risk factors for inflammatory bowel diseases: Evidence based literature review. World J Gastroenterol 2016;22:6296-317. [Crossref] [PubMed]
- Li S, Wang Y, Wang F, et al. A New Perspective for Parkinson's Disease: Circadian Rhythm. Neurosci Bull 2017;33:62-72. [Crossref] [PubMed]
- Schmidt FM, Kirkby KC, Lichtblau N. Inflammation and Immune Regulation as Potential Drug Targets in Antidepressant Treatment. Curr Neuropharmacol 2016;14:674-87. [Crossref] [PubMed]
- Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med 2017;15:131. [Crossref] [PubMed]
- Münzel T, Sørensen M, Gori T, et al. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J 2017;38:557-64. [PubMed]
- Meng L, Ding Y, Li J, et al. Impact of inflammatory markers on the relationship between sleep quality and diabetic kidney disease. Sleep Breath 2021; [Epub ahead of print]. [PubMed]
- Ruszkowski J, Heleniak Z, Król E, et al. Associations between symptoms of constipation and sleep quality in patients with nondialysis chronic kidney disease: a cross-sectional study. Pol Arch Intern Med 2021;131:512-9. [PubMed]
- Küçük O, Kaynar K, Arslan FC, et al. Comparison of mental health, quality of sleep and life among patients with different stages of chronic kidney disease and undergoing different renal replacement therapies. Hippokratia 2020;24:51-8. [PubMed]
- Santiapillai J, Tadtayev S, Miles A, et al. Dihydropyridine calcium channel blockers and obstructive sleep apnea: Two underrecognized causes of nocturia? Neurourol Urodyn 2020;39:1612-4. [Crossref] [PubMed]
- Alvaro PK, Roberts RM, Harris JK. A Systematic Review Assessing Bidirectionality between Sleep Disturbances, Anxiety, and Depression. Sleep 2013;36:1059-68. [Crossref] [PubMed]
- Gress-Smith JL, Roubinov DS, Andreotti C, et al. Prevalence, severity and risk factors for depressive symptoms and insomnia in college undergraduates. Stress Health 2015;31:63-70. [Crossref] [PubMed]
- Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep 1999;22:S354-8. [PubMed]
- Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry 1996;39:411-8. [Crossref] [PubMed]
- Lovato N, Gradisar M. A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep Med Rev 2014;18:521-9. [Crossref] [PubMed]
- Doane LD, Gress-Smith JL, Breitenstein RS. Multi-method assessments of sleep over the transition to college and the associations with depression and anxiety symptoms. J Youth Adolesc 2015;44:389-404. [Crossref] [PubMed]
- Adams SK, Kisler TS. Sleep quality as a mediator between technology-related sleep quality, depression, and anxiety. Cyberpsychol Behav Soc Netw 2013;16:25-30. [Crossref] [PubMed]
- Galvez-Sánchez CM, Montoro CI, Duschek S, et al. Depression and trait-anxiety mediate the influence of clinical pain on health-related quality of life in fibromyalgia. J Affect Disord 2020;265:486-95. [Crossref] [PubMed]
- Campos-Fernández D, Fonseca E, Olivé-Gadea M, et al. The mediating role of epileptic seizures, irritability, and depression on quality of life in people with epilepsy. Epilepsy Behav 2020;113:107511. [Crossref] [PubMed]
- He S, Zhu J, Jiang W, et al. Sleep disturbance, negative affect and health-related quality of life in patients with maintenance hemodialysis. Psychol Health Med 2019;24:294-304. [Crossref] [PubMed]
- Fang H, Tu S, Sheng J, et al. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med 2019;23:2324-32. [Crossref] [PubMed]
- Plante DT. The Evolving Nexus of Sleep and Depression. Am J Psychiatry 2021;178:896-902. [Crossref] [PubMed]
- Natale P, Ruospo M, Saglimbene VM, et al. Interventions for improving sleep quality in people with chronic kidney disease. Cochrane Database Syst Rev 2019;5:CD012625. [Crossref] [PubMed]
- Saeedi P, Salpea P, Karuranga S, et al. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2020;162:108086.


