
Systematic review and meta-analysis on the influence of thyroid dysfunction in early pregnancy on pregnancy outcomes under ultrasound guidance
Introduction
Thyroid disease is a common clinical endocrine disease, with a high incidence in women of childbearing age (1). During pregnancy, the immune status of pregnant women changes and massive amounts of hormones are secreted, which affects the production and metabolism of the thyroid gland, leading to thyroid dysfunction, and ultimately causing adverse effects on maternal and newborn health (2). Thyroid hormone plays an extremely important role in human growth, differentiation, development, and maintaining metabolic balance (3). Thyroid hormones have two main forms, triiodothyronine (T3) and tetraiodothyronine (T4). Changes in the physiological state of pregnant women can cause changes in thyroid function within the normal range during pregnancy. This change is mainly manifested in that with the increase of gestational weeks, serum free T3 (FT3) and free T4 (FT4) levels gradually decrease, while thyroid stimulating hormone (TSH) level gradually increases (4). Human chorionic gonadotropin (hCG) is an important factor leading to changes in thyroid function during pregnancy. During non-pregnancy, the concentration of hCG in the blood is extremely low, but the level of hCG gradually reaches its peak after one week in the first trimester, which promotes the secretion of thyroid hormones, thereby feedback inhibiting the secretion of TSH and reducing the concentration of TSH in pregnant women (5). In the second and third trimesters of pregnancy, maternal basal metabolic rate increases, thyroid hormone consumption increases, glomerular excretion rate increases, urinary iodine excretion increases, and fetal growth requires increased iodine demand, which can lead to relative insufficiency of thyroid hormones. In addition, negative feedback of the hypothalamic-pituitary-thyroid axis increases serum TSH (6). Thyroid hormones not only affect the development and maturation of the fetal meridian system, but are mainly involved in the occurrence, multiplication and migration of neuronal tissues (7). Excessive or insufficient secretion of thyroid hormones can lead to imbalances in the endocrine system of the human body and affect sex hormones and gonadal function (8). Thyroid hormone deficiency leads to immaturity of neuronal tissue and severely affects brain function (9). At present, thyroid serological indicators and thyroid ultrasound are mainly used to diagnose thyroid dysfunction during pregnancy. Serum is the main indicator for the diagnosis of hypothyroidism, and the diagnosis of subclinical hypothyroidism is completely dependent on the serum TSH value. However, due to changes in the physiological state of pregnant women, there is currently no unified standard for the reference range of TSH during pregnancy (10). Thyroid ultrasound has been widely used in the diagnosis of thyroid dysfunction during pregnancy due to its non-invasive, non-radiative, simple operation, and low-cost features (11).
Thyroid dysfunction during pregnancy mainly includes hypothyroidism, hyperthyroidism, thyroid adenoma, thyroid nodule, and goiter (12). Hypothyroidism has an insidious onset, and the clinical symptoms and manifestations of patients vary (13). Hypothyroidism occurs in up to 2.5% of pregnancies, and pregnancy with hypothyroidism is associated with abortion, premature delivery, stillbirth, placental abruption, gestational hypertension, and other adverse pregnancy outcomes (14). In addition, hypothyroidism in the first trimester of pregnancy has a certain impact on the intelligence and cognitive ability of newborns (15). Subclinical hypothyroidism can lead to impaired cardiovascular function and abnormal lipid levels. Walsh et al. [2016] found that the incidence of subclinical hypothyroidism in early pregnancy is 2.3% (16). Moleti et al. [2019] also showed that the prevalence of subclinical hypothyroidism in pregnant women is as high as 11.3% (17). The incidence of pregnancy with hyperthyroidism ranges from 0.5% to 1% (18), leading to adverse pregnancy outcomes such as abortion, premature delivery, stillbirth, gestational hypertension, placental abruption, and premature rupture of membranes (19). Levie et al. [2018] (20) used meta-analysis to explore the effect of low maternal free thyroxine on children’s IQ and autism, and the results pointed out that maternal low free thyroxine has a certain correlation with children’s IQ and autism. Low free thyroxine mainly results in transient hypoalbuminemia of hypothyroidism (primary) pituitary or painless subacute thyroiditis. Therefore, this work mainly analyzed the effect of hypothyroidism on children’s IQ and autism. However, a systematic evaluation of the influence of thyroid dysfunction in early pregnancy on pregnancy outcomes has not yet been reported.
To systematically evaluate the influence of thyroid dysfunction in early pregnancy on pregnancy outcomes, meta-analysis was used to evaluate the difference in pregnancy outcomes between patients with normal and abnormal thyroid function in early pregnancy. This work aimed to provide medical evidence for the clinical evaluation of the influence of thyroid dysfunction in early pregnancy on pregnancy outcomes. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-47/rc).
Methods
Patients and included data
Women who were diagnosed with thyroid diseases such as hypothyroidism, hyperthyroidism, thyroid adenoma, thyroid nodule, and goiter by ultrasonography in the endocrinology department and gynecology department were selected as the research subjects. The types of studies included were retrospective controlled studies and prospective cohort studies. The control group included pregnant women with normal thyroid function. The data included the study author, year, country, number of subjects, age of subjects, and observation indicators.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) articles published between January 2010 and June 2021; (II) clinical randomized controlled studies, cohort studies, or case-control studies based on the grouping of normal and abnormal thyroid function; (III) research subjects included more than 1 case of a pregnant woman in early pregnancy; (IV) the abnormal group had abnormal thyroid function, while the normal group had normal thyroid function; (V) basic information such as age, gender, grouping of patients, the ratio of primiparas, anemia, intrauterine growth restriction, perinatal fetal death, premature delivery, fetal distress syndrome, Apgar score less than 7 within 1 min, and incidence of neonatal complications in the normal and abnormal groups were recorded and counted in detail.
The exclusion criteria were as follows: (I) individual case reports, literature reviews, and non-randomized controlled trials; (II) non-English literature and animal studies; (III) no original data was provided; (IV) repeated publications.
Retrieval strategy
In PubMed (2010–6/2021), Nature (2010–6/2021), Web of Science (2010–6/2021), Springer (2010–6/2021), and Science Direct (2010–6/2021), the following keywords were searched: “thyroid dysfunction”, “thyroid disorder”, “early trimester of pregnancy”, “ultrasound”, “pregnancy outcome”, “hypothyroidism”, “thyroid adenoma”, and “meta-analysis”. Keywords were searched together after being set as “or” and “and”. Clinical studies published between 2010 and June 2021 on the effect of thyroid dysfunction in early pregnancy on pregnancy outcomes were searched. All search keywords were freely combined and input into each database to search the target literature. The publication time of the retrieved literature was set as January 1, 2010 to June 1, 2021. All searches were without language restriction.
Literature selection and quality evaluation
Based on the Cochrane system, the quality of the included literature was evaluated, and the literature was extracted separately by 2 reviewers so as to exclude literature that did not meet the requirements and were of low quality. When the evaluation results were inconsistent, it was necessary for the 2 reviewers to decide whether to include the literature through discussion, or a third reviewer conducted the final evaluation.
The bias of the included literature was evaluated using Cochrane Review Manual 4.2.6. The evaluation criteria included: (I) whether the research method was correct and clear; (II) whether the study clearly explained the random sequence generation method; (III) whether the research results were clear and definite, whether the data were complete, and whether there was a problem of selective reporting; (IV) whether there was selective reporting of the results; (V) whether there was blinding of participants and personnel. According to the criteria, the included literature was divided into 3 grades (low bias, medium bias, and high bias). The literature was initially screened by reading the title, and missing data was supplemented by contacting the author of the original text. Further reading of the abstract and full text was performed, then the results after reading were combined with the Jadad scale to evaluate the quality of the included literature. Studies with a Jadad scale score above 3 were included in this meta-analysis. For the selected study, information on all available variables was extracted and entered into a Microsoft Excel database.
Data extraction of the studies
The data of the included studies were extracted by 2 literature reviewers. The main data extracted included: (I) basic information such as title, first author, publication year, journal, research type, and research start and end time; (II) data on subjects such as the number of included samples, age of subjects, body mass index (BMI), and proportion of primiparas; (III) evaluation methods such as statistics and analyses of the main indexes in the abnormal group and normal group; (IV) anemia, gestational hypertension, gestational diabetes mellitus, intrauterine growth restriction, perinatal fetal death, preterm birth, fetal distress syndrome, cesarean section rate, Apgar score <7 within 1 min, and incidence of neonatal complications were used as outcome indicators to analyze the effect of thyroid dysfunction in early pregnancy on pregnancy outcome.
Statistical methods
Excel 2016 was used to sort the data in the included literature, and Cochrane Reviewer’ Handbook and Jadad scale were used to evaluate the literature quality. RevMan 5.3 was used for meta-analysis of the included literature.
For heterogeneity analysis, the Chi-square test was used to preliminarily test the heterogeneity of the literature. The test significance level was set as α=0.05 and P<0.05. I2 in RevMan 5.3 was used for quantitative evaluation of the heterogeneity results. When I2<25%, there was low heterogeneity. When 25%<I2<50%, there was medium heterogeneity. When I2>50%, there was high heterogeneity. Based on this, when I2<50%, the fixed effects model was used for meta-analysis. When I2>50%, the random effects model was used for meta-analysis.
Relative risk (RR), odds ratio (OR), and risk difference (RD) were used to describe dichotomous variables. Weighted mean difference (WMD) or standard mean difference (SMD) were used to describe continuous variables. Sensitivity analysis was performed by excluding articles with the lowest quality scores. RevMan 5.3 was used to output the forest plots, and Z and P values in the results were extracted to judge the results of meta-analysis. Each effect size was expressed by a 95% confidence interval (CI). When P<0.05, the difference between groups was statistically significant.
Results
Literature retrieval process
After keywords were searched in PubMed and other databases, 1,893 articles were obtained, and 191 related studies were included after preliminary screening of repeated articles. There were 94 articles retrieved from the PubMed database, 38 from the Web of Science database, 25 from the Springer database, 11 from Nature, and 13 from the Science Direct database. In addition, 10 articles were retrieved from literature reviews. After excluding literature that did not meet the inclusion criteria, 65 articles were obtained, and reviews, conference articles, case analyses, and risk factor assessments were excluded based on the title, abstract, and research content. After preliminary screening, a total of 19 articles met the inclusion criteria. After further intensive reading of the articles, 6 articles without access to original data and uncontrolled studies were excluded. Finally, 13 studies were included for meta-analysis (21-33). The literature retrieval and screening process is illustrated in Figure 1.
Basic information of the included literature
A total of 788,867 pregnant women were included in the 13 studies, including 777,458 normal thyroid function cases and 112,299 abnormal thyroid function cases. The basic information of the included literature is illustrated in Table 1.
Table 1
| First author | Year | Country | Research design | Sample size | Normal group (cases) | Abnormal group (cases) |
|---|---|---|---|---|---|---|
| Ajmani SN (21) | 2014 | India | Retrospective comparative study | 400 | 347 | 53 |
| Arbib N (22) | 2017 | Israel | Retrospective comparative study | 4,504 | 3,231 | 1,273 |
| Feldthusen AD (23) | 2014 | Denmark | Retrospective comparative study | 113 | 94 | 19 |
| Hirsch D (24) | 2013 | Israel | Retrospective comparative study | 306 | 205 | 101 |
| Karakosta P (25) | 2012 | Greece | Prospective cohort study | 1,141 | 914 | 227 |
| Kiran Z (26) | 2021 | Pakistan | Retrospective comparative study | 292 | 119 | 173 |
| Konar H (27) | 2018 | India | Retrospective comparative study | 64 | 38 | 26 |
| Kumru P (28) | 2015 | Turkey | Prospective cohort study | 395 | 264 | 131 |
| Luewan S (29) | 2011 | Thailand | Prospective cohort study | 540 | 360 | 180 |
| Männistö T (30) | 2013 | America | Retrospective comparative study | 223,512 | 216,901 | 6,611 |
| Sahu MT (31) | 2010 | India | Prospective cohort study | 633 | 552 | 81 |
| Turunen S (32) | 2020 | Finland | Retrospective comparative study | 553,004 | 550,860 | 2,144 |
| Zhang Y (33) | 2019 | China | Prospective cohort study | 3,783 | 3,573 | 210 |
The normal group had normal thyroid function, and the abnormal group had abnormal thyroid function.
Quality evaluation of the included literature
The Cochrane Reviewer’ Handbook was used for quality evaluation of the 13 included studies, and evaluation charts were drawn for overall evaluation of literature quality. The results are shown in Figures 2,3. The results of the literature quality evaluation using the Cochrane Reviewer’ Handbook were all above grade B. Subsequently, the Jadad scale was used to evaluate the quality of the included literature, and it was found that the Jadad scale scores were all greater than 3 points, so sensitivity analysis was not required.
Analysis of the proportion of primiparas in the 2 groups of pregnant women
Comparative analysis was performed on the proportion of primiparas included in the 7 studies, as illustrated in Figure 4. The heterogeneity of the proportion of primiparas among groups was analyzed, and the results showed that there was high heterogeneity in this indicator (I2=97%, P<0.00001). Therefore, random effects model was used for statistical analysis. The results showed that there was no significant heterogeneity in the proportion of primiparas in normal and abnormal thyroid function groups (OR =1.05, 95% CI: 0.73–1.50; Z=0.25, P=0.80).

Comparison of the incidence of pregnancy anemia between the 2 groups
Comparative analysis was performed on the incidence of pregnancy with anemia among the subjects included in the 3 studies, as illustrated in Figure 5. The heterogeneity of the incidence of pregnancy with anemia was analyzed between groups, and the results showed that heterogeneity was not high for this indicator (I2=0%, P=0.89). Therefore, a fixed effects model was used for statistical analysis of the combined effect. The results of the meta-analysis of the combined effect statistical model showed that there was considerable heterogeneity in the incidence of anemia associated with pregnancy in the normal and abnormal thyroid function groups (OR =0.82, 95% CI: 0.70–0.96, Z=2.48, P=0.01). Notably, the incidence of pregnancy anemia in the abnormal thyroid function group was dramatically higher than that in the normal group (P<0.05).
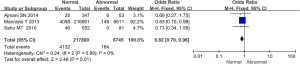
Comparison of the incidence of fetal growth restriction between the 2 groups during pregnancy
Comparative analysis was conducted on the incidence of fetal growth restriction during pregnancy among the subjects included in the 3 studies, as illustrated in Figure 6. The heterogeneity of the incidence of fetal growth restriction in pregnancy between groups was analyzed, and the results showed high heterogeneity (I2=93%, P<0.00001). Therefore, a random effects model was used for statistical analysis of the combined effect. Meta-analysis of the combined effect statistical model showed that there was considerable heterogeneity in the incidence of fetal growth restriction during pregnancy between the normal and abnormal thyroid function groups (OR =0.18, 95% CI: 0.02–1.26, Z=1.73, P=0.08). The incidence of fetal growth restriction in the abnormal thyroid function group was slightly higher than that in the normal group, but there was no significant difference (P>0.05).
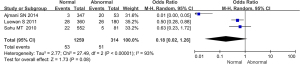
Comparison of perinatal fetal mortality between the 2 groups
Data on perinatal fetal mortality included in 4 studies were compared and analyzed, as illustrated in Figure 7. The heterogeneity of perinatal fetal mortality between groups was analyzed, and the results showed that there was heterogeneity in this indicator (I2=65%, P=0.04). Therefore, a random effects model was used for statistical analysis of the combined effect. Meta-analysis of the combined effect statistical model showed that there was no considerable heterogeneity in perinatal fetal mortality between the normal and abnormal thyroid function groups (OR =0.60, 95% CI: 0.26–1.38, Z=1.20, P=0.23). There was no significant difference in perinatal fetal mortality between the abnormal thyroid function group and normal group (P>0.05).

Comparison of the preterm birth rate between the 2 groups
All 13 studies included detailed statistics on the occurrence of preterm birth in newborns, and the incidence of preterm birth in the 2 groups was compared and analyzed, as illustrated in Figure 8. The heterogeneity of the preterm birth rate between the 2 groups was analyzed, and the results showed that there was high heterogeneity in the preterm birth rate between the 2 groups (I2=96%, P<0.00001). Therefore, a random effects model was used for statistical analysis of the combined effect. Meta-analysis of the combined effect statistical model showed that there was considerable heterogeneity in the incidence of preterm birth in the normal and abnormal thyroid function groups (OR =0.56, 95% CI: 0.36–0.86, Z=2.66, P=0.008). The incidence of preterm delivery in the abnormal thyroid function group was dramatically higher than that in the normal group, and the difference was significant (P<0.05).
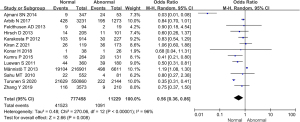
Comparison of the incidence of fetal distress syndrome between the 2 groups
Comparative analysis was conducted on the incidence of fetal distress syndrome among the 7 studies, as illustrated in Figure 9. The heterogeneity of the incidence of fetal distress syndrome was analyzed between groups, and the results showed that there was no heterogeneity in this indicator (I2=0%, P=0.44). Therefore, a fixed effects model was used for statistical analysis of the combined effect. Meta-analysis of the combined effect statistical model showed that there was considerable heterogeneity in the incidence of fetal distress syndrome between the normal and abnormal thyroid function groups (OR =0.76, 95% CI: 0.68–0.85, Z=4.74, P<0.00001). The incidence of fetal distress syndrome in the thyroid dysfunction group was dramatically higher than that in the normal group, and the difference was significant (P<0.05).
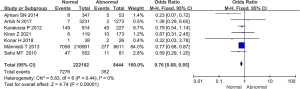
Apgar score between the 2 groups
The proportion of pregnant women with an Apgar score less than 7 within 1 min in 6 studies was compared and analyzed, as illustrated in Figure 10. The heterogeneity of the proportion of pregnant women with an Apgar score less than 7 within 1 min between groups was analyzed. The results showed that there was no heterogeneity in this indicator (I2=0%, P=0.42). Therefore, the fixed effects model was used for statistical analysis of the combined effect. Meta-analysis of the combined effect statistical model showed that there was considerable heterogeneity in the proportion of pregnant women with an Apgar score less than 7 within 1 min in the normal and abnormal thyroid function groups (OR =0.52, 95% CI: 0.34–0.80, Z=2.97, P=0.003). The proportion of pregnant women with an Apgar score less than 7 within 1 min in the thyroid dysfunction group was dramatically higher than that in the normal group, and there was a significant difference between them (P<0.05).
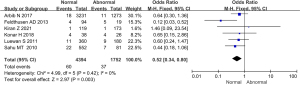
Comparison of the incidence of neonatal complications between the 2 groups
The incidence of neonatal complications in 5 studies was compared and analyzed, and the results are shown in Figure 11. The heterogeneity of the neonatal complication rate between groups was analyzed, and the results showed that there was no heterogeneity in this indicator (I2=22%, P=0.28). Therefore, a fixed effects model was used for statistical analysis of the combined effect. Meta-analysis of the combined effect statistical model showed that there was no considerable heterogeneity in the incidence of neonatal complications between the normal and abnormal thyroid function groups (OR =0.89, 95% CI: 0.79–1.02, Z=1.73, P=0.08). There was no significant difference in the incidence of neonatal complications between the abnormal thyroid function group and the normal group (P>0.05).
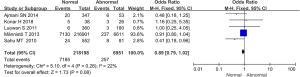
Comparison of the incidence of preeclampsia between the 2 groups
The incidence of preeclampsia among 11 studies was compared and analyzed, as illustrated in Figure 12. Heterogeneity in the incidence of preeclampsia between groups was analyzed, and the results showed that there was no heterogeneity in this indicator (I2=21%, P=0.27). Therefore, a fixed effects model was used for statistical analysis of the combined effect. Meta-analysis of the combined effect statistical model showed that there was considerable heterogeneity in the incidence of preeclampsia between the normal and abnormal thyroid function groups (OR =0.65, 95% CI: 0.48–0.87, Z=2.91, P=0.004). The incidence of preeclampsia in the thyroid dysfunction group was dramatically higher than that in the normal group, with a significant difference (P<0.05).
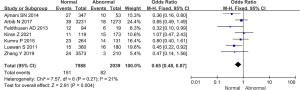
Comparison of the incidence of placental abruption between the 2 groups
The incidence of placental abruption among the included subjects in 11 studies was compared and analyzed, and the results are shown in Figure 13. There was no heterogeneity in the incidence of placental abruption between groups (I2=0%, P=0.86). Therefore, a fixed effects model was used for statistical analysis of the combined effect. Meta-analysis of the combined effect statistical model showed that there was considerable heterogeneity in the incidence of placental abruption between the normal and abnormal thyroid function groups (OR =0.27, 95% CI: 0.19–0.38, Z=7.52, P<0.00001). The incidence of placental abruption in the abnormal thyroid function group was dramatically higher than that in the normal group (P<0.05).
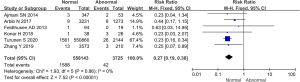
Comparison of the incidence of postpartum hemorrhage between the 2 groups
Comparative analysis was performed on the incidence of postpartum hemorrhage among the subjects included in 4 studies, and the results are shown in Figure 14. The heterogeneity of the incidence of postpartum hemorrhage between groups was analyzed, and the results showed that there was no heterogeneity in this indicator (I2=0%, P=0.51). Therefore, a fixed effects model was used for statistical analysis of the combined effect. Meta-analysis of the combined effect statistical model showed that there was considerable heterogeneity in the incidence of postpartum hemorrhage between the normal and abnormal thyroid function groups (OR =0.62, 95% CI: 0.42–0.92, Z=2.38, P=0.02). The incidence of postpartum hemorrhage in the abnormal thyroid function group was dramatically higher than that in the normal group (P<0.05).
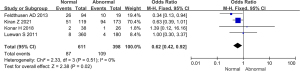
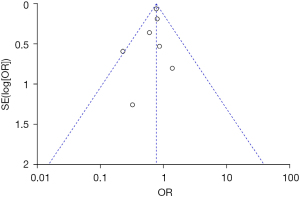
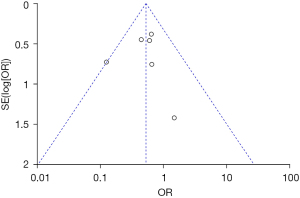
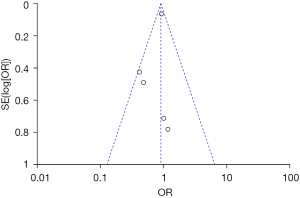
Analysis of publication bias
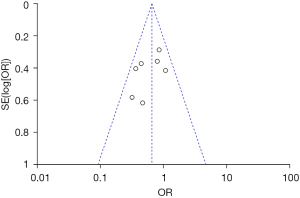
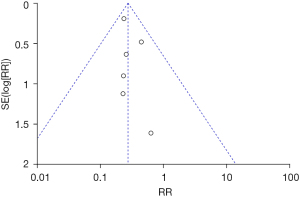
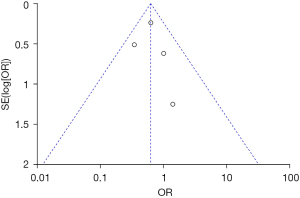
Inverted funnel plots were drawn to analyze the publication bias of the included studies in terms of the indicators of pregnancy outcomes in pregnant women in the normal and abnormal thyroid function groups, as illustrated in Figures 15-21. The inverted funnel plots of all the included outcome indicators were symmetrical, and almost all the included studies fell within the inverted funnel plots. On the whole, each index was close to the central axis. This indicated that the publication bias of all the indexes used for analysis in the included studies was low and met the requirements. Therefore, there was no publication bias and location bias in this study.

Discussion
Changes in the physiological state of a pregnant woman can cause changes in thyroid function within the normal range during pregnancy (34). Such changes mainly manifest as gradual decreases in the serum levels of free T3 and free thyroxine with the increase of gestational age, while the level of TSH gradually increases (8). In recent years, many studies have suggested that the incidence of thyroid dysfunction in pregnant women is dramatically high and is related to changes in maternal and fetal physiological hormone metabolism during pregnancy (35,36). Thyroid dysfunction not only leads to pregnancy-induced hypertension, diabetes, anemia, and other diseases in pregnant women, but also affects the development of the fetal nervous system to some extent (37). During pregnancy, timely detection of thyroid dysfunction and coordinated control by appropriate planning are helpful for reducing the occurrence of adverse pregnancy outcomes. Once pregnant women are complicated with thyroid dysfunction during pregnancy, their thyroid function is highly susceptible to the influence of chorionic gonadotropin, thyrotropin-releasing hormone, and various drugs secreted by the placenta (38). In the 13 studies included in this meta-analysis, there were no statistically significant differences in age, BMI, and the proportion of primiparas between the abnormal thyroid function group and the normal group (P>0.05), indicating that the baseline data was comparable. The results showed that the incidence of hypertension and diabetes in pregnant women with abnormal thyroid function was dramatically higher than that in the normal group, suggesting that abnormal thyroid function can cause the occurrence of hypertension and diabetes in pregnancy. At present, the specific mechanism of gestational hypertension caused by thyroid dysfunction is not clear. The reason may be that decreased cardiac output in pregnant women leads to changes in peripheral circulation resistance, resulting in increased sympathetic excitability caused by secondary epinephrine, eventually leading to hypertension (39). Abnormal glucose metabolism is one of the risk factors for subclinical hypothyroidism during pregnancy, and abnormal thyroid function during pregnancy can lead to an increased incidence of diabetes (40).
Preterm birth is defined as delivery between 28- and 37-week gestation (41). Our results showed that the incidence of preterm birth in the thyroid dysfunction group was dramatically higher than that in the normal group. Current studies point to endocrine factors as one of the causes of preterm birth (42), as well as chromosomal abnormalities, anatomical abnormalities, hereditary and acquired thrombocytopenia, environmental exposure, and immune factors. Hypothyroidism can be caused by inadequate or deficient thyroid hormone synthesis, secretion, or biological effects. Insufficient thyroid hormone synthesis can affect the function of trophoblast cells in the placenta and reduce the endocrine function of trophoblast cells, which can easily cause the occurrence of premature labor (43). After 20 weeks of gestation or during delivery, the placenta in its normal position is partially or completely removed from the uterine wall before delivery (44). The specific mechanism of placental abruption induced by thyroid dysfunction remains unclear. Postpartum hemorrhage is defined as blood loss with related signs or hypovolemic symptoms that occur within 24 hours of delivery, with a blood loss of 1,000 mL or more at 24 hours postpartum (45). The results in this study showed that thyroid dysfunction could increase the incidence of postpartum hemorrhage. In conclusion, women with abnormal thyroid function and pregnancy, untreated or poorly controlled, have a higher risk of maternal and fetal complications. However, if the thyroid function can be basically controlled in the normal range throughout the pregnancy, the pregnancy outcome is usually good. At present, it is often treated with drugs. The goal of treatment of thyroid dysfunction is to maintain TSH and control FT4 to the upper limit of normal (46). The dose of thyroxine replacement therapy needs to be increased with increasing gestational age (47). Therefore, in the treatment of hypothyroidism and pregnancy, the earlier the treatment is started, the better.
This meta-analysis explored the influence of thyroid dysfunction in early pregnancy, as determined by ultrasound, on pregnancy outcomes. The results showed that the incidence of anemia, hypertension, fetal growth restriction, diabetes, preterm birth, fetal distress syndrome, cesarean section, preeclampsia, placental abruption, and postpartum hemorrhage in pregnant women with thyroid dysfunction were dramatically higher than those in the normal group. However, low birth weight, neonatal jaundice, abortion, and neonatal complications were not associated with thyroid dysfunction.
Conclusions
A meta-analysis was conducted to explore the influence of thyroid dysfunction in early pregnancy on adverse pregnancy outcomes. We found that thyroid dysfunction was associated with anemia, hypertension, fetal growth restriction, diabetes mellitus, premature delivery, fetal distress syndrome, cesarean section, preeclampsia, placental abruption, and postpartum hemorrhage. However, there are still some limitations in this study. Only 3 studies were included in the analysis of the occurrence of abortion, and the results may be biased to some extent. In the future, further clinical trials should be conducted to analyze the correlation between abortion and thyroid dysfunction. In conclusion, thyroid dysfunction in early pregnancy is strongly associated with a variety of adverse pregnancy outcomes. This work provides a theoretical basis for the early diagnosis of thyroid dysfunction in pregnant women.
Acknowledgments
Funding: This work was supported by the 2019 Hebei Province Medical Science Research Key Project Plan (No. 20191450).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-47/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-47/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krysiak R, Szkróbka W, Okopień B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto's Thyroiditis: A Pilot Study. Exp Clin Endocrinol Diabetes 2019;127:417-22. [Crossref] [PubMed]
- Costantine MM, Smith K, Thom EA, et al. Effect of Thyroxine Therapy on Depressive Symptoms Among Women With Subclinical Hypothyroidism. Obstet Gynecol 2020;135:812-20. [Crossref] [PubMed]
- Jabbar A, Pingitore A, Pearce SH, et al. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol 2017;14:39-55. [Crossref] [PubMed]
- Gauthier BR, Sola-García A, Cáliz-Molina MÁ, et al. Thyroid hormones in diabetes, cancer, and aging. Aging Cell 2020;19:e13260. [Crossref] [PubMed]
- Carvalho DP, Dupuy C. Thyroid hormone biosynthesis and release. Mol Cell Endocrinol 2017;458:6-15. [Crossref] [PubMed]
- Persani L, Campi I. Syndromes of Resistance to Thyroid Hormone Action. Exp Suppl 2019;111:55-84. [Crossref] [PubMed]
- Nazarpour S, Ramezani Tehrani F, Simbar M, et al. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol 2017;176:253-65. [Crossref] [PubMed]
- Maraka S, Mwangi R, McCoy RG, et al. Thyroid hormone treatment among pregnant women with subclinical hypothyroidism: US national assessment. BMJ 2017;356:i6865. [Crossref] [PubMed]
- Wang H, Gao H, Chi H, et al. Effect of Levothyroxine on Miscarriage Among Women With Normal Thyroid Function and Thyroid Autoimmunity Undergoing In Vitro Fertilization and Embryo Transfer: A Randomized Clinical Trial. JAMA 2017;318:2190-8. [Crossref] [PubMed]
- Ren B, Wan S, Liu L, et al. Distributions of serum thyroid-stimulating hormone in 2020 thyroid disease-free adults from areas with different iodine levels: a cross-sectional survey in China. J Endocrinol Invest 2021;44:1001-10. [Crossref] [PubMed]
- Arikan TA. Plasma Selenium Levels in First Trimester Pregnant Women with Hyperthyroidism and the Relationship with Thyroid Hormone Status. Biol Trace Elem Res 2015;167:194-9. [Crossref] [PubMed]
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017;27:315-89. [Crossref] [PubMed]
- De Leo S, Pearce EN. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol 2018;6:575-86. [Crossref] [PubMed]
- Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ 2019;365:l2006. [Crossref] [PubMed]
- Angell TE, Alexander EK. Thyroid Nodules and Thyroid Cancer in the Pregnant Woman. Endocrinol Metab Clin North Am 2019;48:557-67. [Crossref] [PubMed]
- Walsh JP. Managing thyroid disease in general practice. Med J Aust 2016;205:179-84. [Crossref] [PubMed]
- Moleti M, Di Mauro M, Sturniolo G, et al. Hyperthyroidism in the pregnant woman: Maternal and fetal aspects. J Clin Transl Endocrinol 2019;16:100190. [Crossref] [PubMed]
- Andersen SL, Knøsgaard L. Management of thyrotoxicosis during pregnancy. Best Pract Res Clin Endocrinol Metab 2020;34:101414. [Crossref] [PubMed]
- Andersen SL, Andersen S. Turning to Thyroid Disease in Pregnant Women. Eur Thyroid J 2020;9:225-33. [Crossref] [PubMed]
- Levie D, Korevaar TIM, Bath SC, et al. Thyroid Function in Early Pregnancy, Child IQ, and Autistic Traits: A Meta-Analysis of Individual Participant Data. J Clin Endocrinol Metab 2018;103:2967-79. [Crossref] [PubMed]
- Ajmani SN, Aggarwal D, Bhatia P, et al. Prevalence of overt and subclinical thyroid dysfunction among pregnant women and its effect on maternal and fetal outcome. J Obstet Gynaecol India 2014;64:105-10. [Crossref] [PubMed]
- Arbib N, Hadar E, Sneh-Arbib O, et al. First trimester thyroid stimulating hormone as an independent risk factor for adverse pregnancy outcome. J Matern Fetal Neonatal Med 2017;30:2174-8. [Crossref] [PubMed]
- Feldthusen AD, Larsen J, Pedersen PL, et al. Pregnancy-induced alterations in mitochondrial function in euthyroid pregnant women and pregnant women with subclinical hypothyroidism; relation to adverse outcome. J Clin Transl Endocrinol 2013;1:e13-7. [Crossref] [PubMed]
- Hirsch D, Levy S, Nadler V, et al. Pregnancy outcomes in women with severe hypothyroidism. Eur J Endocrinol 2013;169:313-20. [Crossref] [PubMed]
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012;97:4464-72. [Crossref] [PubMed]
- Kiran Z, Sheikh A, Islam N. Association of thyroid antibodies status on the outcomes of pregnant women with hypothyroidism (maternal hypothyroidism on pregnancy outcomes, MHPO-4). BMC Pregnancy Childbirth 2021;21:136. [Crossref] [PubMed]
- Konar H, Sarkar M, Roy M. Association of Thyroid Dysfunction and Autoimmunity in Pregnant Women with Diabetes Mellitus. J Obstet Gynaecol India 2018;68:283-8. [Crossref] [PubMed]
- Kumru P, Erdogdu E, Arisoy R, et al. Effect of thyroid dysfunction and autoimmunity on pregnancy outcomes in low risk population. Arch Gynecol Obstet 2015;291:1047-54. [Crossref] [PubMed]
- Luewan S, Chakkabut P, Tongsong T. Outcomes of pregnancy complicated with hyperthyroidism: a cohort study. Arch Gynecol Obstet 2011;283:243-7. [Crossref] [PubMed]
- Männistö T, Mendola P, Reddy U, et al. Neonatal outcomes and birth weight in pregnancies complicated by maternal thyroid disease. Am J Epidemiol 2013;178:731-40. [Crossref] [PubMed]
- Sahu MT, Das V, Mittal S, et al. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Gynecol Obstet 2010;281:215-20. [Crossref] [PubMed]
- Turunen S, Vääräsmäki M, Lahesmaa-Korpinen AM, et al. Maternal hyperthyroidism and pregnancy outcomes: A population-based cohort study. Clin Endocrinol (Oxf) 2020;93:721-8. [Crossref] [PubMed]
- Zhang Y, Li Y, Shan Z, et al. Association of Overt and Subclinical Hyperthyroidism During Weeks 4-8 with Adverse Pregnancy Outcomes. J Womens Health (Larchmt) 2019;28:842-8. [Crossref] [PubMed]
- Stagnaro-Green A, Dong A, Stephenson MD. Universal screening for thyroid disease during pregnancy should be performed. Best Pract Res Clin Endocrinol Metab 2020;34:101320. [Crossref] [PubMed]
- Lee S, Farwell AP. Euthyroid Sick Syndrome. Compr Physiol 2016;6:1071-80. [Crossref] [PubMed]
- Springer D, Jiskra J, Limanova Z, et al. Thyroid in pregnancy: From physiology to screening. Crit Rev Clin Lab Sci 2017;54:102-16. [Crossref] [PubMed]
- Shan Z, Teng W. Thyroid hormone therapy of hypothyroidism in pregnancy. Endocrine 2019;66:35-42. [Crossref] [PubMed]
- Wildisen L, Feller M, Del Giovane C, et al. Effect of Levothyroxine Therapy on the Development of Depressive Symptoms in Older Adults With Subclinical Hypothyroidism: An Ancillary Study of a Randomized Clinical Trial. JAMA Netw Open 2021;4:e2036645. [Crossref] [PubMed]
- Safian S, Esna-Ashari F, Borzouei S. Thyroid Dysfunction in Pregnant Women with Gestational Diabetes Mellitus. Curr Diabetes Rev 2020;16:895-9. [Crossref] [PubMed]
- López-Muñoz E, Mateos-Sánchez L, Mejía-Terrazas GE, et al. Hypothyroidism and isolated hypothyroxinemia in pregnancy, from physiology to the clinic. Taiwan J Obstet Gynecol 2019;58:757-63. [Crossref] [PubMed]
- Andersen SL, Andersen S, Liew Z, et al. Maternal thyroid disease and adiposity in mother and child. Clin Endocrinol (Oxf) 2021;94:484-93. [Crossref] [PubMed]
- Ollero MD, Pineda J, Martínez de Esteban JP, et al. Optimization of the follow-up of pregnant women with autoimmune thyroid disease. Endocrinol Diabetes Nutr (Engl Ed) 2019;66:305-11. [Crossref] [PubMed]
- Shokri S, Hekmatnia A, Farghadani M, et al. Thyroid volume and nodular and diffuse thyroid diseases by ultrasonography in pregnant women: A case-control study. J Res Med Sci 2020;25:13. [Crossref] [PubMed]
- Sepasi F, Rashidian T, Shokri M, et al. Thyroid dysfunction in Iranian pregnant women: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2020;20:405. [Crossref] [PubMed]
- Guo W, Wang W, Jin Y, et al. Trimester-Specific Thyroid Function in Pregnant Women with Different Iodine Statuses. Ann Nutr Metab 2020;76:165-74. [Crossref] [PubMed]
- Kurimoto C, Inaba H, Ariyasu H, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci 2020;111:1468-77. [Crossref] [PubMed]
- Diederichsen SZ, Darkner S, Chen X, et al. Short-term amiodarone treatment for atrial fibrillation after catheter ablation induces a transient thyroid dysfunction: Results from the placebo-controlled, randomized AMIO-CAT trial. Eur J Intern Med 2016;33:36-41. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)





