
Efficacy and safety of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers in diabetic nephropathy: a systematic review and meta-analysis
Introduction
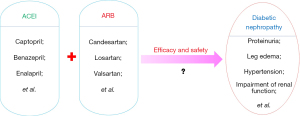
Diabetic nephropathy (DN) is not only one of the most typical microangiopathies caused by diabetes, but is also an important cause of end-stage renal disease. Patients with diabetes complicated with this disease often show persistent albuminuria, lower limb edema, hypertension, and various progressive renal dysfunction (1-3), which not only imposes physical and psychological burdens on patients’ premature delivery, but also seriously affects their quality of life. Therefore, adoption of reasonable drug treatment is valuable. At present, the treatment of such diseases mainly includes angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) (see Figure 1). ACEI drugs, such as captopril, benazepril, and enalapril, can achieve therapeutic effects by inhibiting ACE, preventing the transformation of (angiotensin I) Ang I to (angiotensin II) Ang II, so as to reduce the production of Ang II (4,5). ARB drugs, such as candesartan, losartan, and valsartan, can block the binding of Ang II to receptor 1 (AT1), thereby reducing the biological activity of Ang II to achieve therapeutic benefits (6,7). Both of these drug types can act on different levels of Renin-Angiotensin-Aldosterone System (RAAS). However, for patients with advanced DN, it is often difficult to achieve the clinical effect of protecting the kidneys via ACEI or ARB monotherapy(8). Therefore, an increasing number of clinical researchers began to explore new drug treatment schemes on this basis, and consistently investigated the clinical effect of ACEI and ARB combined treatment (9,10). In this study, we performed a meta-analysis to evaluate the efficacy and safety of ACEIs and ARBs in the treatment of DN. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-212/rc).
Methods
Data sources and search strategy
The PubMed, Embase, Web of Science, and Medline databases were selected as the data sources for this study. The literature search was limited to studies published in English. The literature search methods involved a rapid search of English words as well as a combinatorial search of literature keywords. The keywords were as follows: “angiotensin converting enzyme inhibitors”, “angiotensin II receptor antagonist”, “diabetic nephropathy”, “combined medication”, “clinical efficacy”, “safety”, and “randomized controlled trial”. A free combination of these keywords was utilized for database full-text retrieval. At the same time, the relevant citations were tracked by manual retrieval. The literature search and retrieval were conducted on November 20, 2021.
Inclusion criteria
The inclusion criteria were as follows: (I) randomized controlled trials evaluating the clinical efficacy and safety of ACEIs and ARBs in the treatment of DN; (II) articles with no lack of data on the clinical efficacy and safety of combined treatment of ACEIs and ARBs in DN; (III) the intervention measures involved the administration of ACEI and ARB combined treatment in the test group patients, and ACEI or ARB single-drug treatment for patients in the control group. If the control group was given ACEI plus placebo or ARB plus placebo, it was considered to be equivalent to ACEI or ARB single-drug treatment; (IV) studies involving subjects diagnosed with DN. Patients in the treatment and control groups received regular diet control and oral hypoglycemic agents or insulin therapy to control blood glucose, accompanied by persistent microalbuminuria, and other secondary renal diseases were excluded; and (V) articles that included the main outcome indicators set out in this study.
Exclusion criteria
The exclusion criteria were as follows: (I) studies involving non-DN patients; (II) non-randomized controlled trials; (III) articles with incomplete analysis data; (IV) repeatedly published studies; and (V) studies involving early DN patients with non-microalbuminuria.
Selection of literature
Two researchers independently performed the literature screening. Firstly, the titles and abstracts of the articles were read to exclude studies that obviously did not meet the inclusion requirements. Subsequently, we read the full texts of the remaining literatures for further screening. Next, two researchers performed cross-checking to exclude doubtful articles. Lastly, a third researcher was invited to assist in arbitration.
Data extraction
Two researchers independently extracted the relevant data and information, including the first author, publication time, trial design, intervention measures, follow-up time, endpoint events, sample size, as well as the average age, sex ratio, and duration of disease of patients. All data were independently extracted and analyzed by two authors. In cases of divergent opinions, a comprehensive evaluation was conducted by a third party.
Literature quality assessment
The Newcastle-Ottawa scale (NOS score method) was used for treatment evaluation of the included articles, with higher scores indicating better quality literature and less bias. We found that four articles scored 5 points, two articles scored 4 points, and two articles scored 2 points, which was of good quality.
Statistical analysis
RevMan5.3 software provided by Cochrane Collaboration Network was used for meta-analysis. The weighted mean difference (MD) was used for measurement data, the odds ratio (OR) and 95% CI were used for counting data to report the size of combined effect, and forest maps were used to display the results. Also, the Q statistic test was used to examine the heterogeneity among the studies; if there was no statistical heterogeneity (P>0.1, I2≤50%), the fixed effects model is used to analyze the data. However, if there was statistical heterogeneity (P≤0.1, I2>50%), the data included in the study was preliminarily analyzed and evaluated. We then judged whether there was obvious research heterogeneity or methodological heterogeneity by reading the full texts of the included literature several times. Subsequently, sensitivity analysis was carried out on the outcome indicators of each study one-by-one, and new data were merged after excluding unqualified studies. Finally, we checked whether the overall effect amount had changed. If the result after each elimination was the same as the previous total consolidation result, this indicates that the result was relatively stable. However, if the aforementioned methods could not explain the causes of heterogeneity, the data was combined and analyzed using a random effects model. P<0.05 was considered statistically significant for all the above effect analyses.
Results
Literature search and screening results
In total, 457 articles were initially retrieved. 199 repetitive literatures, 91 ineligible literatures, and 30 other irrelevant studies were removed. After reading the titles and abstracts of the remaining studies, 102 articles that did not meet the inclusion criteria were removed. Furthermore, 18 reviews and articles with incomplete data were excluded after intensive reading. The references of the included studies were also searched and read. Finally, eight articles were included, with a total of 1,893 DN patients (Figure 2). The basic and common characteristics of the included studies, as well as the excluded literature and the reasons for exclusion are listed in Table 1.
Table 1
Basic characteristics of literatures
The basic characteristics and NOS scores of the included articles are shown in Table 2.
Table 2
| Serial number | Author | Study location | Date of publication | Total cases | Efficacy and safety | Quality score (points) |
|---|---|---|---|---|---|---|
| 1 | Titan SM et al. (15) | Brazil | 2011 | 56 | (I)(II)(III)(V)(VII) | 5 |
| 2 | Sengul AM et al. (16) | Istanbul, Turkey | 2006 | 192 | (II)(III)(IV)(V)(VI) | 5 |
| 3 | Jacobsen P et al. (17) | Denmark | 2002 | 24 | (I)(IV)(VI)(VI)(VIII) | 5 |
| 4 | Tan F et al. (18) | Singapore | 2010 | 34 | (I)(II)(III)(V) | 4 |
| 5 | Song JH et al. (19) | Republic of Korea | 2006 | 21 | (I)(IV)(V) (VI)(VII) | 5 |
| 6 | Fernandez Juarez G et al. (20) | Oviedo, Spain | 2013 | 133 | (IV)(VI)(VII) | 3 |
| 7 | Rossing K et al. (21) | Denmark | 2003 | 20 | (I)(IV)(VI) | 3 |
| 8 | Saglimbene V et al. (22) | Sydney, Australia | 2018 | 1,413 | (I)(IV)(VII)(IX) | 4 |
(I) 24 h proteinuria; (II) systolic blood pressure; (III) diastolic blood pressure; (IV) serum creatinine; (V) creatinine clearance; (VI) serum potassium; (VII) adverse event; (VIII) glomerular filtration rate; (IX) hyperkalemia.
Meta-analysis results
Efficacy
Twenty-four-hour proteinuria
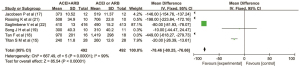
Six articles(15,17,18,19,21,22) reported the clinical effect of ACEI and ARB combination therapy on the 24-hour proteinuria in patients with DN. There were 492 cases in the combined drug group and 492 cases in the single drug group. There was heterogeneity among the literatures (I2=99%, P<0.00001). No cause of heterogeneity was identified through analysis, so the random effect model was used for data analysis. The results showed that the urinary protein in the combined drug group was lower than that in the single drug group within 24 hours, and the difference was statistically significant (MD =−78.46, 95% CI: −80.25 to −76.66, P<0.00001), as shown in Figure 3.
Systolic blood pressure
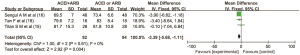
Three articles (15,16,18) reported the clinical effect of the combined use of ACEIs and ARBs on systolic blood pressure in patients with DN. There were 92 cases in the combined drug group and 94 cases in the single drug group. There was no heterogeneity between the literatures (I2=0%, P=0.69), so the fixed effect model was used for data analysis. The results showed that the clinical index of systolic blood pressure in the combined ACEI and ARB group was lower than that in the ACEI or ARB monotherapy group, and the difference was statistically significant (MD =−9.11, 95% CI: −13.44 to −4.78, P<0.0001) as shown in Figure 4.

Diastolic blood pressure
Three articles (15,16,18) reported the clinical effect of combination therapy of ACEI and ARB on diastolic blood pressure in patients with DN. There were 92 cases in the combined drug group and 94 cases in the single drug group. There was no heterogeneity among the articles (I2=0%, P=0.61), so the fixed effect model was used for data analysis. The results showed that the clinical index of diastolic blood pressure in the combined ACEI and ARB group was lower than that in the ACEI or ARB alone group, and the difference was statistically significant (MD =−3.39, 95% CI: −5.68 to −1.11, P=0.004), as shown in Figure 5.
Safety
Serum creatinine
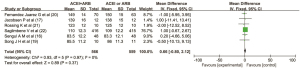
Six articles(16,17,19,20,21,22) reported the effect of combined use of ACEI and ARB on serum creatinine in patients with DN. There were 566 cases in the combined drug group and 559 cases in the single drug group. There was no heterogeneity among the literatures (I2=0%, P=0.97), so the fixed effect model was used for data analysis. The results showed that there was no significant difference in the clinical index of diastolic blood pressure between the ACEI and ARB combined group and the ACEI or ARB monotherapy group (MD =0.66, 95% CI: −0.80 to 2.12, P=0.37), as shown in Figure 6.
Creatinine clearance
Four articles(15,16,18,19) reported the effect of the combined use of ACEI and ARB on the creatinine clearance rate in patients with DN. There were 102 cases in the combined drug group and 105 cases in the single drug group. There was heterogeneity among the literatures (I2=80%, P=0.002). No cause of heterogeneity was found through analysis, so the random effect model was used for data analysis. The results showed that there was no significant difference in the clinical index of creatinine clearance between ACEI and ARB combined group and ACEI or ARB monotherapy group (MD =−0.25, 95% CI: −0.62 to 0.11, P=0.17), as shown in Figure 7.
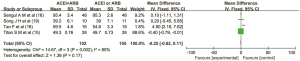
Serum potassium
Five articles (16,17,19,20,21)reported the effect of the combined use of ACEI and ARB on serum potassium in patients with DN. There were 150 cases in the combined drug group and 144 cases in the single drug group. There was heterogeneity among the literatures (I2=70%, P=0.01). No cause of heterogeneity was found through analysis, so the random effect model was used for data analysis. The results showed that the serum potassium level in the combined drug group was lower than that in the single drug group, and the difference was statistically significant (MD =0.10, 95% CI: 0.05 to 0.15, P=0.0001), as shown in Figure 8 below.
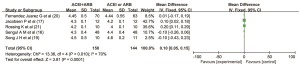
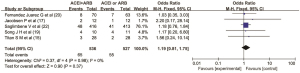
Adverse events
Five articles (15,17,19,20,22) reported the adverse reactions of ACEIs and ARBs in patients with DN. There were 536 cases in the combined drug group and 527 cases in the single drug group. There was no heterogeneity among the literatures (I2=0%, P=0.98), so the fixed effect model was used for data analysis. The results showed that there was no significant difference in the incidence of adverse reactions between the ACEI and ARB combined group and the ACEI or ARB monotherapy group (OR =1.19, 95% CI: 0.81 to 1.75, P=0.37), as shown in Figure 9.
Publication bias
Publication bias analysis was not performed, as only a small number of articles were included in this study.
Discussion
DN is one of the most common complications in diabetic patients. Its early pathological changes are mainly characterized by thickening of the glomerular basement membrane, increased permeability, proliferation of mesangial cells, and abnormal increase of extracellular matrix, leading to glomerular sclerosis in patients (23,24). If these patients do not receive effective means of clinical intervention and drug treatment, their condition may easily develop into the clinical stage of DN. At this time, patients will not only be accompanied by a large amount of proteinuria, but also have clinical symptoms of hypertension and severe renal function impairment. Therefore, it has high research significance to improve the physiological status and quality of life of patients with DN through effective intervention (25,26).
At present, treatment of DN includes glucose lowering, lipid lowering, blood pressure control, administrative micro blog, anti-oxidative stress, and renal function (27). ACEI or ARB drugs are widely used in treatment. Clinical studies have confirmed that these two drug types can not only effectively reduce the excretion of urinary protein and reduce the level of blood pressure, but also have high safety (28). However, the clinical efficacy and safety of these two drugs in the treatment of DN are still in the exploratory stage (29). Therefore, this systematic analysis and evaluation of existing research materials/data is important for further clinical guidance.
The results of this systematic review and meta-analysis suggest that in terms of effectiveness of drug use, patients with DN who received combined therapy with ACEIs and ARBs had significantly lower 24 h urine protein, systolic blood pressure, and diastolic blood pressure than those treated with ACEIs or ARBs alone, and the difference between the two groups was statistically significant (P<0.05). In terms of safety, in addition to the significant difference in serum potassium between the combined ACEI and ARB group and the single-drug group (P<0.05), there were no significant differences in serum creatinine, creatinine clearance, and the incidence of adverse reactions (P>0.05).
Conclusions
In this meta-analysis of the clinical efficacy and safety of ACEIs and ARBs in patients with DN, a total of eight articles were included. The results showed that the combination of ACEIs and ARBs had better clinical effects on the control of 24-hour urine protein, systolic blood pressure, and diastolic blood pressure in patients with DN, as well as higher safety and a lower incidence of adverse events.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-212/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-212/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum 2018. Am J Kidney Dis 2018;71:884-95. [Crossref] [PubMed]
- Shimizu M, Furuichi K, Wada T. Epidemiology and pathogenesis of diabetic nephropathy. Nihon Jinzo Gakkai Shi 2017;59:43-9. [PubMed]
- Najafian B, Alpers CE, Fogo AB. Pathology of human diabetic nephropathy. Contrib Nephrol 2011;170:36-47. [Crossref] [PubMed]
- Montinaro V, Cicardi M. ACE inhibitor-mediated angioedema. Int Immunopharmacol 2020;78:106081. [Crossref] [PubMed]
- Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibi-tor-induced angioedema: A review of the literature. J Clin Hypertens (Greenwich) 2017;19:1377-82. [Crossref] [PubMed]
- Timmermans PB. Pharmacological properties of angiotensin II receptor antagonists. Can J Cardiol 1999;15 Suppl F:26F-8F.
- Gleiter CH, Jägle C, Gresser U, et al. Candesartan. Cardiovasc Drug Rev 2004;22:263-84. [Crossref] [PubMed]
- Nadolnik K, Skrypnik D, Skrypnik K, et al. Diabetic nephropathy in the elderly – clinical practice Rocz Panstw Zakl Hig 2018;69:327-34. [Crossref] [PubMed]
- Taal MW, Brenner BM. Combination ACEI and ARB therapy: additional benefit in renoprotection? Curr Opin Nephrol Hypertens 2002;11:377-81. [Crossref] [PubMed]
- MacKinnon M, Shurraw S, Akbari A, et al. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic re-view of the efficacy and safety data. Am J Kidney Dis 2006;48:8-20. [Crossref] [PubMed]
- Krairittichai U, Chaisuvannarat V. Effects of dual blockade of renin-angiotensin sys-tem in type 2 diabetes mellitus patients with diabetic nephropathy. J Med Assoc Thai 2009;92:611-7. [PubMed]
- Stanton RC. Combination use of angiotensin converting enzyme inhibitors and angi-otensin receptor blockers in diabetic kidney disease. Curr Diab Rep 2013;13:567-73. [Crossref] [PubMed]
- Luno J, Praga M, de Vinuesa SG. The reno-protective effect of the dual blockade of the renin angiotensin system (RAS). Curr Pharm Des 2005;11:1291-300. [Crossref] [PubMed]
- Rodriguez F, Lee DJ, Gad SS, et al. Real-World Diagnosis and Treatment of Diabetic Kidney Disease. Adv Ther 2021;38:4425-41. [Crossref] [PubMed]
- Titan SM. ACEI and ARB combination therapy in patients with macroalbuminuric diabetic nephropathy and low socioeconomic level: a double-blind randomized clinical trial. Clin Nephrol 2011;76:273-83. [Crossref] [PubMed]
- Sengul AM, Altuntas Y, Kürklü A, et al. Beneficial effect of lisinopril plus telmisar-tan in patients with type 2 diabetes, microalbuminuria and hypertension. Diabetes Res Clin Pract 2006;71:210-9. [Crossref] [PubMed]
- Jacobsen P, Andersen S, Rossing K, et al. Dual blockade of the renin-angiotensin system in type 1 patients with diabetic nephropathy. Nephrol Dial Transplant 2002;17:1019-24. [Crossref] [PubMed]
- Tan F, Mukherjee JJ, Lee KO, et al. Dual blockade of the ren-in-angiotensin-aldosterone system is safe and effective in reducing albuminuria in Asian type 2 diabetic patients with nephropathy. Singapore Med J 2010;51:151-6. [PubMed]
- Song JH, Cha SH, Lee HJ, et al. Effect of low-dose dual blockade of ren-in-angiotensin system on urinary TGF-beta in type 2 diabetic patients with advanced kidney disease. Nephrol Dial Transplant 2006;21:683-9. [Crossref] [PubMed]
- Fernandez Juarez G, Luño J, Barrio V, et al. Effect of dual blockade of the ren-in-angiotensin system on the progression of type 2 diabetic nephropathy: a random-ized trial. Am J Kidney Dis 2013;61:211-8. [Crossref] [PubMed]
- Rossing K, Jacobsen P, Pietraszek L, et al. Renoprotective effects of adding angio-tensin II receptor blocker to maximal recommended doses of ACE inhibitor in dia-betic nephropathy: a randomized double-blind crossover trial. Diabetes Care 2003;26:2268-74. [Crossref] [PubMed]
- Saglimbene V, Palmer SC, Ruospo M, et al. The Long-Term Impact of Ren-in-Angiotensin System (RAS) Inhibition on Cardiorenal Outcomes (LIRICO): A Randomized, Controlled Trial. J Am Soc Nephrol 2018;29:2890-9. [Crossref] [PubMed]
- Furuichi K, Shimizu M, Okada H, et al. Clinico-pathological features of kidney dis-ease in diabetic cases. Clin Exp Nephrol 2018;22:1046-51. [Crossref] [PubMed]
- Ritz E, Zeng XX, Rychlík I. Clinical manifestation and natural history of diabetic nephropathy. Contrib Nephrol 2011;170:19-27. [Crossref] [PubMed]
- Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, pre-vention, and treatment. Diabetes Care 2005;28:164-76. [Crossref] [PubMed]
- A/L B Vasanth Rao VR. Diabetic nephropathy: An up-date on pathogenesis and drug development. Diabetes Metab Syndr 2019;13:754-62. [Crossref] [PubMed]
- Sternlicht H, Bakris GL. Management of Hypertension in Diabetic Nephropathy: How Low Should We Go? Blood Purif 2016;41:139-43. [Crossref] [PubMed]
- Kandhare AD, Mukherjee A, Bodhankar SL. Antioxidant for treatment of diabetic nephropathy: A systematic review and meta-analysis. Chem Biol Interact 2017;278:212-21. [Crossref] [PubMed]
- Gallagher H, Suckling RJ. Diabetic nephropathy: where are we on the journey from pathophysiology to treatment? Diabetes Obes Metab 2016;18:641-7. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



