
Factors affecting sarcopenia in older patients with chronic diseases
Introduction
Sarcopenia, first identified by Rosenberg in 1997, is a clinical syndrome characterized by decreased muscle mass and function, and is closely related to aging (1). Sarcopenia compromises independence and quality of life, increases the risks of falls, fractures, disability, and death, and is associated with high medical expenses. Sarcopenia will be a major health problem of the future, and has received increased attention within the academic community. The prevalence of sarcopenia in older adults varies greatly by population characteristics, disease status, and the diagnostic criteria and measurement tools employed. The prevalence rates are between 5% and 25%, ranging from 5–13% in community-dwelling older people aged 65 years and above, rising to 20–25% in those aged 80 years and older (2). Despite the high prevalence, the rate of awareness and diagnosis of sarcopenia before problems such as physical dysfunction or falls occur, is relatively low. In other words, the condition of sarcopenia in elderly adults are often overlooked.
The pathogenesis of sarcopenia is multifactorial. The condition is related to a variety of chronic diseases including chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), cardiovascular and cerebrovascular conditions, and metabolic endocrine diseases (3-5). Most previous studies on sarcopenia have been based on community-dwelling older adults (6-8), while those studies based on hospitalized patients have focused more on the association between sarcopenia and their adverse clinical outcomes (9-11). Many studies (12,13) have shown that sarcopenia in older patients with chronic diseases often predicts poor prognosis. There are a few studies which have comprehensively analyzed the associated factors, such as bone parameters, hormone levels, inflammatory status and nutritional factors. Here, we analyzed the factors affecting sarcopenia in older patients with chronic diseases, and compared such patients to those without sarcopenia over the same period. We sought to establish a basis and direction for further research on the associated factors of sarcopenia, aiming to the early and timely screening of sarcopenia and define therapeutic interventions that counteract the muscle atrophy and functional decline associated with aging, which will ultimately reduce the incidence of sarcopenia and the associated healthcare costs of older adults. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-201/rc).
Methods
Subject selection
This was a case-control study. A total of 121 older patients with chronic diseases admitted to the Department of Geriatrics of Affiliated Kunshan Hospital of Jiangsu University from May 2019 to April 2021 were enrolled, and (using appropriate diagnostic criteria) divided into a sarcopenia group (n=57) and a non-sarcopenia group (n=64). The sarcopenia group included 34 males and 23 females, while the non-sarcopenia group included 23 males and 41 females. Application of the sarcopenia diagnostic criteria formulated by the Asian Working Group for Sarcopenia (AWGS) (14) requires: (I) determination of skeletal muscle mass [we used dual-energy X-ray absorptiometry (DXA)]. Appendicular lean mass (ALM) served as an index of relative muscle mass. The sums of the ALMs of the upper and lower limbs, divided by the square of the height (ALM/height2, the relative skeletal muscle index (RSMI)), were calculated. We used the adjusted Asian population standard. Males with scores of <7.00 kg/m2 and females with scores of <5.4 kg/m2 were considered to exhibit muscle mass declines. (II) Muscle strength measurement: For males, a handgrip strength <28 kg was defined as low, while the figure for females was <18 kg. (III) Physical performance measurements: the 6-min normal walking time and the 5-time chair stand test were used to evaluate physical performance; a walking speed ≤1.0 m/s and a 5-time chair stand test time ≥12 s served as the cut-offs for low physical performance. If the above criteria were met, sarcopenia was diagnosed. When criterion 1 and either criterion 2 or 3 were met, this indicated sarcopenia. When all 3 criteria were met, sarcopenia was severe.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Affiliated Kunshan Hospital of Jiangsu University (No. 2021-01-011-H01). Individual consent for this retrospective analysis was waived.
Outcome variables
General data
Two geriatric specialists reviewed the medical records of all patients, including data on age, gender, body fat percentage, body mass index (BMI), basic activities of daily living (assessed using the Barthel Index), and accompanying chronic diseases (diabetes mellitus, hypertension, cardiovascular and cerebrovascular diseases, peripheral arterial disease, COPD, and malignant tumors), which were then compared between the 2 groups.
Bone mineral density (BMD) and body composition
The BMDs of lumbar spine vertebrae L1-4 and the left hip (the femoral neck) were measured via DXA, and T-values were calculated. Whole-body muscle and adipose tissue levels were simultaneously assessed. Osteoporosis was considered present when the BMD T-value of the lumbar spine or femoral neck was ≤−2.5.
Laboratory findings
All patients fasted for more than 8 h overnight and venous blood was collected in the morning. An automated blood cell analyzer was used to measure hemoglobin levels. A biochemical analyzer was employed to evaluate liver function (prealbumin and albumin levels). Atomic absorption spectrometry was used to determine the serum levels of trace elements. The electrochemiluminescence method was employed to assess serum 25 hydroxyvitamin D levels. Folic acid (FA) and vitamin B12 levels were measured by radioimmunoassay (RIA), and enzyme-linked immunosorbent assays (ELISAs) were used to measure the levels of hormones and inflammatory cytokines.
Statistical analysis
SPSS and GraphPad Prism statistical software were used for statistical analyses and graph construction, respectively. The normality of distributions was assessed with the Shapiro-Wilk (SW) test. Data that were normally distributed were compared using the independent-samples t-test. A nonparametric test was employed to compare non-normally distributed data. Continuous data are expressed as means ± standard deviations (means ± SDs) and skewed data as medians with interquartile ranges. The chi-squared or Fisher’s exact test was used to compare categorical data. Pearson correlation coefficients between normally distributed data and Spearman correlation coefficients between non-parametric data were derived to analyze associations between sarcopenia-related variables and the RSMI, handgrip strength, and walk speed. Two-sided P values were used in this study and P<0.05 was considered statistically significant.
Results
We included 121 hospitalized patients with various chronic diseases. Their basic characteristics are listed in Table 1. The average age of patients with sarcopenia was significantly higher than that of the non-sarcopenia group (P<0.001). There were 34 males and 23 females in the sarcopenia group, and 23 males and 41 females in the non-sarcopenia group. The gender difference was statistically significant (P<0.01), suggesting that the incidence of sarcopenia in males was higher than that in females with chronic diseases. Sarcopenia patients exhibited a significantly lower BMI and Barthel index (both P<0.001), suggestive of poorer nutritional status and daily function. The incidence of malignant tumors and cognitive impairment in the sarcopenia group was higher than in the non-sarcopenia group (both P<0.05), as was the incidence of COPD and cerebrovascular disease (both P<0.01), but not the incidence of other common chronic diseases.
Table 1
| Variable | Sarcopenia (n=57) | Non-sarcopenia (n=64) | P value |
|---|---|---|---|
| Average age (years) | 76 [70–81] | 67 [59–71] | <0.001 |
| Male percentage | 34 (59.6%) | 23 (35.9%) | 0.009 |
| BMI (kg/m2) | 20.9 (19.0–24.7) | 23.55 (22.3–26.3) | <0.001 |
| Body fat (%) | 29.4 (24.0–36.1) | 32.3 (27.25–37.25) | 0.146 |
| Barthel index | 95 [65–100] | 100 [100–100] | <0.001 |
| Chronic disease | |||
| Diabetes mellitus | 18 | 15 | 0.414 |
| Hypertension | 30 | 34 | 1 |
| COPD | 22 | 7 | 0.001 |
| Cardiovascular disease | 6 | 5 | 0.754 |
| Cerebrovascular disease | 19 | 6 | 0.001 |
| Peripheral arterial disease | 19 | 23 | 0.849 |
| Connective tissue disease | 5 | 7 | 0.768 |
| Chronic atrophic gastritis | 3 | 4 | 1 |
| Malignant tumor | 8 | 2 | 0.045 |
| Cognitive impairment | 4 | 0 | 0.046 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
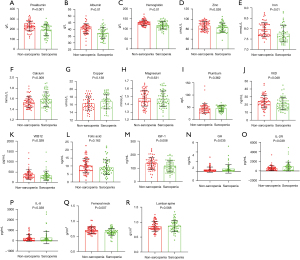
The levels of nutritional markers, hormones, and inflammatory cytokines in the 2 groups are shown in Figure 1. The average hemoglobin level of the sarcopenia group was significantly lower than that of the non-sarcopenia group. The sarcopenia group also exhibited significantly lower levels of albumin and prealbumin (both P<0.001) than the non-sarcopenia group. In terms of trace elements, the serum iron and zinc levels in the sarcopenia group were significantly lower than in the non-sarcopenia group, but the serum calcium level was higher (P<0.05). The serum magnesium, copper, and lead levels did not differ between the 2 groups. The vitamin D and vitamin B12 levels in the sarcopenia group were lower than in the non-sarcopenia group (both P<0.05). The FA levels did not differ between the 2 groups. The serum levels of growth hormone (GH), insulin-like growth factor-1 (IGF-1), and interleukins IL-8 and IL-2R were measured on admission. The serum IGF-1 level in the sarcopenia group was significantly lower than in the non-sarcopenia group (P<0.01), while the GH level was higher (P<0.05). The IL-8 and IL-2R levels were higher in the sarcopenia group (both P<0.05), suggesting that inflammation was associated with sarcopenia.
We then compared BMD between the 2 groups. In the sarcopenia group, 31 patients had osteoporosis compared to 23 in the non-sarcopenia group, and the incidence in the former group was significantly higher (P<0.05). The femoral neck BMD in the sarcopenia group was lower than that in the non-sarcopenia group (P<0.01), but the BMDs of the lumbar spine did not differ between the 2 groups.
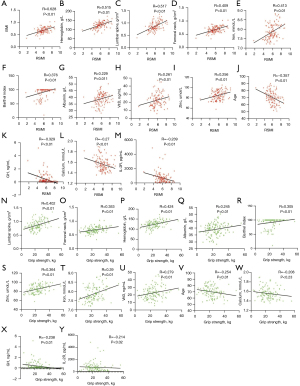
We investigated the correlations between sarcopenia-related variables and the RSMI, handgrip strength, and walk speed. The RSMI exhibited a moderate positive correlation with BMI, BMD, serum hemoglobin and iron levels, and muscle mass (Figure 2A-2E), and mild positive correlations with the Barthel index and the serum levels of albumin, zinc, and vitamin D (Figure 2F-2I). However, age and the levels of serum GH, calcium, and IL-2R were inversely associated with the RSMI (Figure 2J-2M). Grip strength was positively correlated with the BMD (both lumbar spine and femoral neck) and serum hemoglobin, albumin, Barthel index, zinc, iron, as well as vitamin D levels (Figure 2N-2U), and was negatively correlated with age and the serum levels of calcium, GH, and IL-2R (Figure 2V-2Y), which was different from what we originally expected. We found no significant correlation between any of these parameters and walk speed.
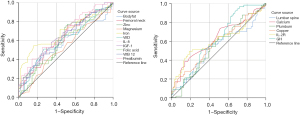
In an effort to predict sarcopenia, all statistically significant variables mentioned above were subjected to further analysis. The ability of these variables to predict sarcopenia is shown in Figure 3. Age predicted sarcopenia well (Figure 3A), with an optimal cutoff of 73.5 years, which yielded a sensitivity of 61.4% and a specificity of 92.2%. Thus, patients older than 73.5 years require careful assessment and a possible diagnosis of sarcopenia. The albumin and hemoglobin levels and the BMI (Figure 3B-3D) were moderate predictors of sarcopenia. Patients with low values of these indicators should be evaluated in terms of sarcopenia. The prealbumin, iron, zinc, vitamin D, and vitamin B12 levels were very weakly predictive (Figure 4).

Discussion
Sarcopenia is considered to be a geriatric syndrome. With increasing age, muscle attenuation and loss are very common, accompanied by osteoporosis, malnutrition, and metabolic syndrome. Sarcopenia in older adults is closely associated with a myriad of poor health outcomes including but not limited to falls, fractures, hospitalization, disability, and all-cause mortality. Sarcopenia has become a hot topic in the field of geriatric medicine.
Early-stage sarcopenia may not be accompanied by symptoms, or perhaps only by fatigue and weight loss. Therefore, it is easily overlooked, not diagnosed, and misdiagnosed. Many medical institutions do not even perform routine screening, and systematic clinical research is lacking. When sarcopenia becomes severe, activity declines, daily activities become difficult, the fall and fracture risks rise, and the outcomes are always adverse. However, sarcopenia can be cured if identified and treated early. Therefore, a comprehensive understanding of the clinical characteristics, risk factors, underlying mechanisms, and factors leading to adverse prognosis is essential.
Sarcopenia is caused by many intrinsic and extrinsic factors. The risk factors include age, gender, a low physical activity level, and the presence of chronic disease (15-17). With aging, changes in the levels of GH, testosterone, thyroxine, and IGF adversely affect muscle quality and strength (18). In addition, poor nutrition and reduced protein synthesis are common in older adults, and malnutrition can aggravate the decline in muscle function and quality (19). In this study, we found that participants with sarcopenia were more likely to be older and malnourished, and aging and malnutrition may be strong predictors of sarcopenia. Male patients were more susceptible to the condition, consistent with most prior epidemiological studies. Their BMIs were lower, daily living was more arduous, and most had chronic diseases including COPD, cerebrovascular conditions, and cognitive disorders, in line with the findings of most previous clinical studies.
Malnutrition is defined as an imbalance between energy, protein, and other nutrients. It is prevalent in various populations, particularly older adults, due to their lower levels of physical activity and a reduction in energy needs, decreased appetite and food intake, poor function of chewing, digestion, and absorption, cognitive decline, multiple medications, and comorbidity status. Malnutrition and sarcopenia often overlap, negatively affecting body composition, physical function, and clinical outcomes. A substantial body of evidence now links poor nutrition to poor muscle quantity and quality in older age. Malnutrition promotes sarcopenia (20). Clinicians should integrate nutrition assessment with sarcopenia screening for optimal evaluation of these 2 interrelated issues to help improve patients’ clinical outcomes (21). Water, minerals, protein, vitamins, fat, and trace elements are essential. It is possible to screen and assess sarcopenia by assessing BMI, body fat percentage, and biochemical markers (serum albumin, prealbumin, hemoglobin, vitamins, and trace element levels). Patients with lower values were at a significantly greater risk of sarcopenia. BMI correlates positively with the RSMI, and a low BMI is a key risk factor for muscle mass decline. We also found trends toward more anemia and hypoproteinemia in patients with sarcopenia. The hemoglobin, albumin, and prealbumin levels were positively associated with both the RSMI and grip strength, indicating that they may contribute to both muscle quantity and quality, and may predict sarcopenia. The mechanism underlying the associations has not been fully clarified. Nutrition plays an extremely important role in terms of muscle health. Malnourished older persons are at increased risk of sarcopenia because of reduced muscle protein synthesis with age (21). BMI and albumin and prealbumin levels are reliable markers of nutrition. Kim et al. suggested that prealbumin degradation might reduce muscle strength and mass, increasing the risk of sarcopenia (20), which is consistent with our findings. Both albumin and prealbumin are negative acute-phase reactants, the concentrations of which fall during inflammation (22). It is widely accepted that inflammation plays a role in the pathogenesis of sarcopenia. Chronic low-grade inflammation contributes to a loss of muscle mass, strength, and function, affecting both muscle protein breakdown and synthesis via several signaling mechanisms (23), which is in line with our observations. We found that levels of the proinflammatory cytokines IL-8 and IL-2R were elevated in sarcopenia patients, the latter of which has also been confirmed to be associated with immune senescence (24).
Trace elements play key roles in both physiological and pathological processes and metabolic activities. Changes in trace element levels may be associated with loss of muscle mass, reduced muscle strength, and functional decline in aging. No systematic review has yet explored the effects of trace elements on sarcopenia. We found that the serum iron and zinc levels in patients with sarcopenia were significantly lower than in those without sarcopenia. Moreover, the levels of these 2 elements positively correlated with the RSMI, indicating that zinc and iron may play important roles in sarcopenia development and progression. Zinc-containing enzymes include alkaline phosphatase, DNA and RNA polymerases, and carboxypeptidases. These enzymes synthesize nucleic acids and proteins, activate other enzymes, and participate in signaling pathways that affect a variety of physiological and pathological processes (25). Zinc is essential for normal metabolism, growth, and development. Muscle cell proliferation and differentiation are accompanied by increases in protein synthesis and cell size. Both processes require zinc, which is also key in terms of the regulation of anti-inflammatory and antioxidant responses, free-radical scavenging, and autophagy (26-28). Inflammation and autophagy contribute to the muscle atrophy of sarcopenia and other chronic diseases. Zinc deficiency may promote sarcopenia by compromising myocyte proliferation and differentiation, and by interfering with antioxidant responses and autophagy. Iron, which binds oxygen, is key in terms of oxygen supply. Iron is an essential component of the hemoglobin of red blood cells, myoglobin, and mitochondrial enzymes. Iron deficiency seriously compromises muscle function and oxidative metabolism (29,30). Neidlein et al. found that iron deficiency was an independent risk factor for physical and muscle dysfunction, fatigue, and slow recovery in older hospitalized patients (31). Patients who are iron-deficient may suffer from fatigue, lack of energy, and reduced daily activity, all of which exacerbate muscle weakness and reduce muscle mass. Iron homeostasis is essential for normal mitochondrial function. Iron is an important component of several complexes of the electron transport chain, and is thus essential for respiration and ATP production. Iron deficiency reduces the numbers and function of skeletal muscle mitochondria, eventually compromising oxidative metabolism. In addition, when iron is lacking, the iron sulfur content of skeletal muscle mitochondria falls, as do the levels of mitochondrial cytochromes and total oxidative capacity. Mitochondrial protein synthesis and respiratory ability are lost, triggering skeletal muscle weakness and motor decline (30,32). We speculate that iron deficiency may be involved in the pathogenesis of sarcopenia by reducing the synthesis of hemoglobin and myoglobin, inducing reductions in the numbers of skeletal muscle mitochondria, and dysregulating the mitochondrial respiratory chain.
FA, vitamin B12, and vitamin D are essential for the maintenance of immune system health and cellular function. FA, also termed vitamin B9, is a necessary micronutrient. Adequate FA intake is essential to ensure cell division and homeostasis during organ growth, along with tissue development and metabolic renewal (33). Romero et al. found that FA ingestion improved nitric oxide-dependent vasodilation and skeletal muscle blood flow by enhancing the vascular conductance of exercising skeletal muscles, thus improving muscle strength (34). However, we found no significant difference in the FA levels between our 2 groups. This may be explained by dietary habits, additional supplements, or test errors. Vitamin B12 and FA are essential cofactors during the metabolic transformation of homocysteine. Impaired methylation attributable to FA and vitamin B12 deficiency can elevate homocysteine concentrations, in turn decreasing muscle strength and walking speed (35,36). Also, depression and cognitive impairment caused by vitamin B12 deficiency may trigger communication disorders (37), following muscle wasting attributable to decreased physical activity. Few studies have explored the link between vitamin B12 status and sarcopenia, and any interrelationship between vitamin B12 level and skeletal muscle health has not been completely elucidated. We found a significantly lower level of vitamin B12 in patients with sarcopenia, but no significant correlation between vitamin B12 level and muscle mass or muscle strength. The effects of vitamin D on bone and muscles have been widely documented. Vitamin D may crucially affect muscle fiber development. Vitamin D deficiency often accompanies reduced muscle mass (38), and adversely affects muscle strength and bodily function. Vitamin D deficiency is an important risk factor for sarcopenia (39,40). We found that sarcopenic patients had a lower level of vitamin D than controls, and the vitamin D level correlated positively with muscle quantity and quality. This result provides convincing evidence for the view that vitamin D supplementation may be an effective therapeutic treatment for sarcopenia. It is worth mentioning that apart from the between-group difference, the average vitamin D levels in both groups were lower than normal, in line with the prevalence of vitamin D deficiency in the Chinese population.
Both sarcopenia and osteoporosis are common age-associated diseases reflecting different aspects of musculoskeletal atrophy. They can both increase the risk of falls, fractures, functional degradation, and mortality. Muscle and bone tissues are both derived from mesenchymal cells and thus exhibit similar genetic, physiological, and biological characteristics. They are anatomically adjacent, co-operate to drive bodily activities, and are subject to both endocrine and paracrine control. The 2 tissues are subject to the same risk factors and exhibit similar pathogeneses. Some drugs target both tissues as they share certain molecular signaling pathways (41). Some authors have suggested that sarcopenia and osteoporosis are in fact the same disease, termed dysmobility syndrome, and thus combine the 2 tissues into a single therapeutic target (42). Muscle is the principal mechanical stimulant of bone tissue synthesis and metabolism (43). Muscle loading induces a series of biomechanical signals required for bone growth and remodeling. Muscle contraction imparts mechanical stress to bone, triggering osteogenesis (44). Changes in muscle contraction in older adults inevitably reduce bone strength, manifesting as reductions in the numbers of horizontal and vertical trabeculae in cancellous bone, thinning of the bone cortex, and reduced anti-torsion, shear, and bending abilities, triggering osteoporosis and even fracture. The Wnt/β-catenin signaling pathway is the principal regulator of bone and muscle growth and development (45). Muscle may produce certain undefined factors that protect and preserve osteocyte vitality. Muscle factors and fluid shear stress act synergistically to activate the Wnt/β-catenin pathway of osteocytes which promotes bone formation (46,47). We found that the incidence of osteoporosis in the sarcopenia group was significantly higher than in the non-sarcopenia group, and we found a moderate positive correlation between BMD and muscle mass, confirming the close relationship between the 2 diseases. In future, sarcopenia assessment should be considered when seeking to prevent osteoporosis. It is essential to explore the signaling pathways and mechanisms shared by the 2 diseases when developing new treatment methods.
Changes in hormone levels may trigger a variety of endocrine metabolic disorders and may affect the synthesis and catabolism of skeletal muscle. GH and IGF-1 are associated with skeletal muscle loss in older adults. Bian et al. found that limb skeletal muscle mass was positively correlated with GH and IGF-1 levels. After 60 years of age, the levels of GH and IGF-1 gradually decrease, reducing protein synthesis in, and metabolism of, skeletal muscle cells, eventually causing changes in skeletal muscle cell structure and function. Reductions in GH and IGF-1 levels play key roles in terms of the loss of skeletal muscle mass (48). The IGF-1 levels that we report are in line with the above theories, but the GH levels differed significantly, unlike what was found in earlier studies. This may be attributable to our small sample size or laboratory error. The clinical significance thereof, as well as the clinical significance of other endocrine system negative feedback, require further exploration in more subjects.
Our work had certain limitations. First, this was a single-center observational study. The sample size was small and confounding factors were in play. We plan to perform a multi-center study with more subjects. Second, the results of serological tests may be affected by environmental and personal factors. For example, vitamin D levels are affected by season and sunshine, and serum trace element levels may be influenced by daily activities or diet. Caution is appropriate when interpreting our current data, and larger prospective studies are needed. Despite these limitations, our findings suggest that sarcopenia is closely related to aging, and that the incidence in males is higher than in females. Patients with sarcopenia find it difficult to engage in the activities of daily living, and thus require special care. Low BMD is positively correlated with both poor muscle mass and strength. Patients with sarcopenia are more likely to have osteoporosis. The 2 conditions are closely related and should be assessed and managed at the same time. Sarcopenia is often accompanied by poor nutritional status. Consequently, it is also accompanied by a low BMI, low levels of hemoglobin, albumin, and prealbumin, and vitamin and trace element deficiencies (especially of vitamin D, vitamin B12, iron, and zinc), and is closely related to changes in hormone levels and chronic inflammation. Older patients with anemia and hypoproteinemia require further analysis and a possible diagnosis of sarcopenia. These variations may play roles in sarcopenia development and progression, and adversely affect muscle quality and function. These characteristics alert early identification of, screening for, and evaluation of sarcopenia in older patients with chronic diseases. We hope our work will pave the way for further studies on the factors affecting sarcopenia, along with prevention and targeted treatment strategies.
Acknowledgments
Funding: This work was supported and funded by the Neurophysiology Team of Affiliated Kunshan Hospital of Jiangsu University, the Social Development Science and Technology Special Project of Kunshan (KSZ1934) and the Science and Technology Project of Suzhou Health and Family Planning Commission (LCZX201911).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-201/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-201/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-201/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Affiliated Kunshan Hospital of Jiangsu University (No. 2021-01-011-H01). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95-101. [Crossref] [PubMed]
- Bianchetti A, Novelli A. Sarcopenia in the elderly: From clinical aspects to therapeutic options. Geriatric Care 2019;5:23-32. [Crossref]
- Androga L, Sharma D, Amodu A, et al. Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney Int Rep 2017;2:201-11. [Crossref] [PubMed]
- Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care 2016;10:236-41. [Crossref] [PubMed]
- Tsuchida K, Fujihara Y, Hiroki J, et al. Significance of Sarcopenia Evaluation in Acute Decompensated Heart Failure. Int Heart J 2018;59:143-8. [Crossref] [PubMed]
- Pang BWJ, Wee SL, Lau LK, et al. Prevalence and Associated Factors of Sarcopenia in Singaporean Adults-The Yishun Study. J Am Med Dir Assoc 2021;22:885.e1-10. [Crossref] [PubMed]
- Su Y, Hirayama K, Han TF, et al. Sarcopenia Prevalence and Risk Factors among Japanese Community Dwelling Older Adults Living in a Snow-Covered City According to EWGSOP2. J Clin Med 2019;8:291. [Crossref] [PubMed]
- Kim M, Won CW. Prevalence of sarcopenia in community-dwelling older adults using the definition of the European Working Group on Sarcopenia in Older People 2: findings from the Korean Frailty and Aging Cohort Study. Age Ageing 2019;48:910-6. [Crossref] [PubMed]
- Gariballa S, Alessa A. Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr 2013;32:772-6. [Crossref] [PubMed]
- Scheerman K, Meskers CGM, Verlaan S, et al. Sarcopenia, Low Handgrip Strength, and Low Absolute Muscle Mass Predict Long-Term Mortality in Older Hospitalized Patients: An Observational Inception Cohort Study. J Am Med Dir Assoc 2021;22:816-820.e2. [Crossref] [PubMed]
- Sipers WMWH, de Blois W, Schols JMGA, et al. Sarcopenia Is Related to Mortality in the Acutely Hospitalized Geriatric Patient. J Nutr Health Aging 2019;23:128-37. [Crossref] [PubMed]
- Liccini A, Malmstrom TK. Frailty and Sarcopenia as Predictors of Adverse Health Outcomes in Persons With Diabetes Mellitus. J Am Med Dir Assoc 2016;17:846-51. [Crossref] [PubMed]
- Cebron Lipovec N, Schols AM, van den Borst B, et al. Sarcopenia in Advanced COPD Affects Cardiometabolic Risk Reduction by Short-Term High-intensity Pulmonary Rehabilitation. J Am Med Dir Assoc 2016;17:814-20. [Crossref] [PubMed]
- Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300-307.e2. [Crossref] [PubMed]
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 2014;11:177-80. [PubMed]
- Sinclair AJ, Abdelhafiz AH, Rodríguez-Mañas L. Frailty and sarcopenia - newly emerging and high impact complications of diabetes. J Diabetes Complications 2017;31:1465-73. [Crossref] [PubMed]
- Peterson SJ, Mozer M. Differentiating Sarcopenia and Cachexia Among Patients With Cancer. Nutr Clin Pract 2017;32:30-9. [Crossref] [PubMed]
- Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008;9:213-28. [Crossref] [PubMed]
- Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci 2003;58:M911-6. [Crossref] [PubMed]
- Kim H, Suzuki T, Kim M, et al. Incidence and predictors of sarcopenia onset in community-dwelling elderly Japanese women: 4-year follow-up study. J Am Med Dir Assoc 2015;16:85.e1-8. [Crossref] [PubMed]
- Vandewoude MF, Alish CJ, Sauer AC, et al. Malnutrition-sarcopenia syndrome: is this the future of nutrition screening and assessment for older adults? J Aging Res 2012;2012:651570. [Crossref] [PubMed]
- Veeranki S, Tyagi SC. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci 2013;14:15074-91. [Crossref] [PubMed]
- Dalle S, Rossmeislova L, Koppo K. The Role of Inflammation in Age-Related Sarcopenia. Front Physiol 2017;8:1045. [Crossref] [PubMed]
- J Heath J. D Grant M. The Immune Response Against Human Cytomegalovirus Links Cellular to Systemic Senescence. Cells 2020;9:766. [Crossref]
- Olechnowicz J, Tinkov A, Skalny A, et al. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci 2018;68:19-31. [Crossref] [PubMed]
- Alker W, Haase H. Zinc and Sepsis. Nutrients 2018;10:976. [Crossref] [PubMed]
- Himoto T, Masaki T. Associations between Zinc Deficiency and Metabolic Abnormalities in Patients with Chronic Liver Disease. Nutrients 2018;10:88. [Crossref] [PubMed]
- Liuzzi JP, Guo L, Yoo C, et al. Zinc and autophagy. Biometals 2014;27:1087-96. [Crossref] [PubMed]
- Stugiewicz M, Tkaczyszyn M, Kasztura M, et al. The influence of iron deficiency on the functioning of skeletal muscles: experimental evidence and clinical implications. Eur J Heart Fail 2016;18:762-73. [Crossref] [PubMed]
- Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 2001;131:568S-579S; discussion 580S. [Crossref] [PubMed]
- Neidlein S, Wirth R, Pourhassan M. Iron deficiency, fatigue and muscle strength and function in older hospitalized patients. Eur J Clin Nutr 2021;75:456-63. [Crossref] [PubMed]
- Leermakers PA, Remels AHV, Zonneveld MI, et al. Iron deficiency-induced loss of skeletal muscle mitochondrial proteins and respiratory capacity; the role of mitophagy and secretion of mitochondria-containing vesicles. FASEB J 2020;34:6703-17. [Crossref] [PubMed]
- Wagner C. Biochemical role of folate in cellular metabolism. Clinical Research and Regulatory Affairs 2001;18:161-80. [Crossref]
- Romero SA, Gagnon D, Adams AN, et al. Folic acid ingestion improves skeletal muscle blood flow during graded handgrip and plantar flexion exercise in aged humans. Am J Physiol Heart Circ Physiol 2017;313:H658-66. [Crossref] [PubMed]
- Kado DM, Bucur A, Selhub J, et al. Homocysteine levels and decline in physical function: MacArthur Studies of Successful Aging. Am J Med 2002;113:537-42. [Crossref] [PubMed]
- Swart KM, van Schoor NM, Heymans MW, et al. Elevated homocysteine levels are associated with low muscle strength and functional limitations in older persons. J Nutr Health Aging 2013;17:578-84. [Crossref] [PubMed]
- Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser 2013;13:1-45. [PubMed]
- Robinson SM, Reginster JY, Rizzoli R, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr 2018;37:1121-32. [Crossref] [PubMed]
- Szulc P, Duboeuf F, Marchand F, et al. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr 2004;80:496-503. [Crossref] [PubMed]
- McCarthy EK, Kiely M. Vitamin D and muscle strength throughout the life course: a review of epidemiological and intervention studies. J Hum Nutr Diet 2015;28:636-45. [Crossref] [PubMed]
- Bonewald LF, Kiel DP, Clemens TL, et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. J Bone Miner Res 2013;28:1857-65. [Crossref] [PubMed]
- Binkley N, Krueger D, Buehring B. What's in a name revisited: should osteoporosis and sarcopenia be considered components of "dysmobility syndrome?". Osteoporos Int 2013;24:2955-9. [Crossref] [PubMed]
- Sharir A, Stern T, Rot C, et al. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development 2011;138:3247-59. [Crossref] [PubMed]
- Picca A, Calvani R, Manes-Gravina E, et al. Bone-Muscle Crosstalk: Unraveling New Therapeutic Targets for Osteoporosis. Curr Pharm Des 2017;23:6256-63. [Crossref] [PubMed]
- Nielsen BR, Abdulla J, Andersen HE, et al. Sarcopenia and osteoporosis in older people: a systematic review and meta-analysis. Eur Geriatr Med 2018;9:419-34. [Crossref] [PubMed]
- Mo C, Romero-Suarez S, Bonewald L, et al. Prostaglandin E2: from clinical applications to its potential role in bone- muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol 2012;6:223-9. [Crossref] [PubMed]
- Jähn K, Lara-Castillo N, Brotto L, et al. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of β-catenin. Eur Cell Mater 2012;24:197-209; discussion 209-10. [Crossref] [PubMed]
- Bian A, Ma Y, Zhou X, et al. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet Disord 2020;21:214. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


