
Development and validation of a survival prediction model for patients received mechanical ventilation in the intensive care unit: a large sample size cohort from the MIMIC database
Introduction
Mechanical ventilation remains one of the primary management measures for many critically ill patients in intensive care units (ICUs), from scheduled surgical operations to acute organ failure (1). It has been reported that the rate of mechanical ventilation for ICU patients varies from 32.9% to 70% (2-4). However, mechanical ventilation has been associated with high short- and long-term mortality and decreased quality of life for patients (5-7). Therefore, it is important for physicians to identify patients requiring mechanical ventilation who have poor prognoses to improve their quality of life.
Many factors have been identified that affect the prognosis of patients receiving mechanical ventilation. Age and sepsis have been shown to be independently associated with increased mortality in patients needing mechanical ventilation in ICU (8-10). Lee et al. evaluated a cohort of 311 patients who received prolonged acute mechanical ventilation. Their results indicated that a body mass index ≤21 kg/m2 was an independent predictor of decreased survival (11). Several studies have focused on single factors influencing the prognosis of patients who have received mechanical ventilation, and various predictive models have been built to assess patient survival. A nomogram is a graphical tool integrating multiple factors that could be adopted to predict death risk for patients. It is a more intuitive and convenient way to explain this risk to patients or family members in doctor-patient communications (8). It has been increasingly used in clinical research to predict prognosis in various diseases, such as acute pancreatitis and colorectal cancer (12,13). A previous study based on 736 participants proposed a model to predict mortality in patients receiving mechanical ventilation and incorporated age, platelet count, the requirement for vasopressors and hemodialysis, and non-trauma admission (14). However, the authors only assessed mortality within 1 year based on small sample and lack of validity evidence, and did not investigate the performance of the prediction model validated in different subgroups based on the reasons for mechanical ventilation, which may have weakened the evidence from the study. An improved prediction model for these patients should be developed.
Herein, we conducted a nomogram to predict short- and long-term survival (1-month, 3-month, 1-year, and 3-year survival) in patients receiving mechanical ventilation using 16,775 participants from the Medical Information Mart for Intensive Care III (MIMIC-III) database. Furthermore, we conducted the internal and subgroup validations based on the reasons for mechanical ventilation (shock, sepsis, trauma, and other causes) to estimate the predictive performance of the nomogram. We present the following article in accordance with the TRIPOD reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-646/rc).
Methods
Study design and population
We conducted the development and validation of a prognosis predicting model using patients from the MIMIC-III, an openly available clinical database (15). The MIMIC-III database contains comprehensive and high-quality data of well-defined and characterized patients admitted to ICUs at the Beth Israel Deaconess Medical Center between 2001 and 2012. Patients aged ≥18 years old who received mechanical ventilation were included in this study. We analyzed only the last ICU stay for patients admitted to the ICU more than once. In total, 16,775 eligible patients were included in the study. The patients were randomly divided into training (n=11,742) and testing (n=5,033) groups according to a 7:3 ratio using a completely random sampling method. An ethics committee or institutional review board approval was exempted because the data were accessed from the MIMIC, a publicly available database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data extraction
The patient information was collected within 24 h of admission to the ICU. The demographic data of the studied population included age, gender, and ethnicity. Clinical characteristics included ICU type [intensive care unit (ICU), coronary care unit (CCU), cardiac surgery recovery unit (CSRU), medical intensive care unit (MICU), surgical intensive care unit (SICU), and trauma surgical intensive care unit (TSICU)], days of mechanical ventilation, ventilator-associated pneumonia, chronic pulmonary disease (CPD), heart failure (HF), hypertension, sepsis, shock, trauma, atrial fibrillation, liver cirrhosis, diabetes mellitus (DM), respiratory failure, malignant tumor, renal failure, and coronary heart disease (CHD). Laboratory indicators included white blood cell (WBC) count, blood urea nitrogen (BUN), and the fraction of inspiration O2 (FIO2). Indexes of disease severity were estimated by the Sequential Organ Failure Assessment (SOFA) and Glasgow Coma Scale (GCS) scores. The causes of mechanical ventilation were recorded, including sepsis, shock, trauma, and others.
Outcome variables
The primary outcomes of this study were 1-month, 3-month, 1-year, and 3-year survival. The follow-up duration of this study was 3 years. The survival status of patients was recorded at 1-month, 3-month, 1-year, and 3-year after ICU withdrawal. The follow-up was terminated when death occurred during the follow-up period.
Missing values and outliers
There were 47 (0.27%) missing values in WBC, 272 (1.62%) in FIO2, 113 (0.67%) in GCS score, and 4 (0.02%) in SOFA score. The missing values were imputed by multiple imputations. For the outliers in this study, multiple imputations were also applied. There were 493 (2.94%) outliers in WBC, imputed by the median ± 3* interquartile range (9.4±3*5.1). BUN had 740 (4.41%) outliers, filled into the median ± 3* interquartile range (19±3*18). Additionally, a sensitivity analysis was performed on the data set before and after imputation. The results of the sensitivity analysis are presented in Table S1.
Statistical analysis
The statistical analyses were performed using R programmer v.4.0.3 (Institute for Statistics and Mathematics, Vienna, Austria). Measurement data are represented by the mean ± standard deviation (mean ± SD) or median with interquartile spacing [M (Q1, Q3)], and the independent samples t-test or Mann-Whitney U test was used for intergroup comparisons. Count data are described by the number of cases or constituent ratio [N (%)], and the χ2 test or Fisher’s exact test was adopted for intergroup comparisons.
Univariate and multivariate Cox regression analyses were used to explore the predictors of survival and develop the predictive model for patients’ survival using mechanical ventilation therapy. The hazard ratio (HR) was calculated, represented by a 95% confidence interval (CI). The time-dependent receiver operating characteristic (ROC) curves and C-index were adopted to assess the predictive capacity of the nomogram. The areas under the ROC curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated by MedCalc software (MedCalc Software Ltd). The threshold value of AUC was over 0.8, indicating the nomogram had well performance. To further verify the stability and performance of the nomogram, we divided patients into different subgroups based on the reasons for mechanical ventilation, including shock, sepsis, trauma, and other causes. Two-sided P<0.05 was considered statistically significant for all analyses.
Results
Subject characteristics
A total of 16,775 participants were initially obtained from the MIMIC database, divided into training (n=11,742) and testing (n=5,033) sets (Figure 1). In the training set, the average age was 64.04 (47.22, 80.86) years, with 4,785 (40.75%) females and 6,927 (59.25%) males. There were 2,493 (21.23%) patients with specific reasons for mechanical ventilation and 9,749 (78.77%) individuals with other causes. Further detailed analyses of other specific causes for mechanical ventilation found that the main reasons were sepsis (579, 4.07%), shock (616, 4.33%), trauma (240, 1.69%), sepsis + shock (989, 6.95%), sepsis + trauma (17, 0.12%), shock + trauma (41, 0.29%), and sepsis + shock + trauma (11, 0.08%) (Figure 2). Of the 11,742 patients, 7340 (62.51%) survived, and 4402 (37.49%) died, with a median survival time of 1,095.00 (52.00, 1,095.00) days. In the testing set, the average age was 64.38 (47.75, 81.01) years, with 2,023 (40.19%) females and 3,010 (59.81%) males. Among the 5,033 patients, 3,144 (62.47%) survived and 1,889 (37.53%) died. There were no differences between the two sets in age, gender, ethnicity, ICU type, median days of mechanical ventilation, comorbidity, reasons for mechanical ventilation, SOFA score, GCS score, and laboratory values (Table 1).
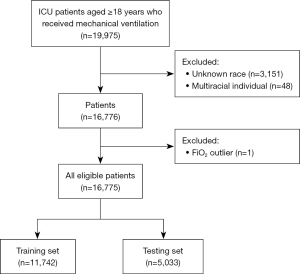
Table 1
| Variables | Total (n=16,775) | Training (n=11,742) | Testing (n=5,033) | Statistics | P |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 64.14±16.76 | 64.04±16.82 | 64.38±16.63 | T=1.18a | 0.237 |
| Gender, n (%) | χ2=0.452b | 0.501 | |||
| Female | 6,808 (40.58) | 4,785 (40.75) | 2,023 (40.19) | ||
| Male | 9,967 (59.42) | 6,957 (59.25) | 3,010 (59.81) | ||
| Ethnicity, n (%) | χ2=1.039b | 0.904 | |||
| Asian | 443 (2.64) | 304 (2.59) | 139 (2.76) | ||
| Black | 1,227 (7.31) | 855 (7.28) | 372 (7.39) | ||
| Hispanic or Latino | 596 (3.55) | 415 (3.53) | 181 (3.60) | ||
| Other | 501 (2.99) | 344 (2.93) | 157 (3.12) | ||
| White | 14,008 (83.51) | 9,824 (83.67) | 4,184 (83.13) | ||
| ICU type, n (%) | χ2=1.181b | 0.881 | |||
| CCU | 1,504 (8.97) | 1,070 (9.11) | 434 (8.62) | ||
| CSRU | 5,276 (31.45) | 3,679 (31.33) | 1,597 (31.73) | ||
| MICU | 4,918 (29.32) | 3,438 (29.28) | 1,480 (29.41) | ||
| SICU | 2,753 (16.41) | 1,931 (16.45) | 822 (16.33) | ||
| TSICU | 2,324 (13.85) | 1,624 (13.83) | 700 (13.91) | ||
| Days of mechanical ventilation | 2.00 (1.00, 5.00) | 2.00 (1.00, 5.00) | 2.00 (1.00, 5.00) | Z=1.201c | 0.230 |
| FIO2 (%), M (Q1, Q3) | 50.00 (40.00, 50.00) | 50.00 (40.00, 50.00) | 50.00 (40.00, 50.00) | Z=1.038c | 0.299 |
| HF, n (%) | χ2=0.003b | 0.957 | |||
| No | 11,997 (71.52) | 8,399 (71.53) | 3,598 (71.49) | ||
| Yes | 4,778 (28.48) | 3,343 (28.47) | 1,435 (28.51) | ||
| CPD, n (%) | χ2=0.573b | 0.449 | |||
| No | 14,338 (85.47) | 10,052 (85.61) | 4,286 (85.16) | ||
| Yes | 2,437 (14.53) | 1,690 (14.39) | 747 (14.84) | ||
| Sepsis, n (%) | χ2=0.141b | 0.708 | |||
| No | 14,494 (86.40) | 10,153 (86.47) | 4,341 (86.25) | ||
| Yes | 2,281 (13.60) | 1,589 (13.53) | 692 (13.75) | ||
| Hypertension, n (%) | χ2=0.020b | 0.886 | |||
| No | 9,195 (54.81) | 6,432 (54.78) | 2,763 (54.90) | ||
| Yes | 7,580 (45.19) | 5,310 (45.22) | 2,270 (45.10) | ||
| Shock, n (%) | χ2=0.013b | 0.908 | |||
| No | 14,424 (85.99) | 10,094 (85.96) | 4,330 (86.03) | ||
| Yes | 2,351 (14.01) | 1,648 (14.04) | 703 (13.97) | ||
| Trauma, n (%) | χ2=0.194b | 0.66 | |||
| No | 16,348 (97.45) | 11,439 (97.42) | 4,909 (97.54) | ||
| Yes | 427 (2.55) | 303 (2.58) | 124 (2.46) | ||
| Atrial fibrillation, n (%) | χ2=0.130b | 0.719 | |||
| No | 11,808 (70.39) | 8,275 (70.47) | 3,533 (70.20) | ||
| Yes | 4,967 (29.61) | 3,467 (29.53) | 1,500 (29.80) | ||
| Liver cirrhosis, n (%) | χ2=1.749b | 0.186 | |||
| No | 15,945 (95.05) | 11,144 (94.91) | 4,801 (95.39) | ||
| Yes | 830 (4.95) | 598 (5.09) | 232 (4.61) | ||
| DM, n (%) | χ2=0.078b | 0.781 | |||
| No | 13,301 (79.29) | 9,317 (79.35) | 3,984 (79.16) | ||
| Yes | 3,474 (20.71) | 2,425 (20.65) | 1,049 (20.84) | ||
| Respiratory failure, n (%) | χ2=0.032b | 0.858 | |||
| No | 11,765 (70.13) | 8,240 (70.18) | 3,525 (70.04) | ||
| Yes | 5,010 (29.87) | 3,502 (29.82) | 1,508 (29.96) | ||
| Malignant tumor, n (%) | χ2=0.001b | 0.977 | |||
| No | 13,383 (79.78) | 9,367 (79.77) | 4,016 (79.79) | ||
| Yes | 3,392 (20.22) | 2,375 (20.23) | 1,017 (20.21) | ||
| Renal failure, n (%) | χ2=0.084b | 0.772 | |||
| No | 12,928 (77.07) | 9,042 (77.01) | 3,886 (77.21) | ||
| Yes | 3,847 (22.93) | 2,700 (22.99) | 1,147 (22.79) | ||
| CHD, n (%) | χ2=0.000b | 0.998 | |||
| No | 10,972 (65.41) | 7,680 (65.41) | 3,292 (65.41) | ||
| Yes | 5,803 (34.59) | 4,062 (34.59) | 1,741 (34.59) | ||
| WBC (k/mL), M (Q1, Q3) | 9.40 (7.20, 12.30) | 9.40 (7.20, 12.30) | 9.40 (7.30, 12.30) | Z=−0.012c | 0.990 |
| BUN (mg/dL), M (Q1, Q3) | 19.00 (13.00, 31.00) | 20.00 (13.00, 31.00) | 19.00 (13.00, 30.00) | Z=−0.720c | 0.472 |
| SOFA score, M (Q1, Q3) | 6.00 (4.00, 8.00) | 6.00 (4.00, 8.00) | 6.00 (4.00, 8.00) | Z=−0.228c | 0.819 |
| GCS score, M (Q1, Q3) | 9.00 (5.00, 14.00) | 9.00 (5.00, 14.00) | 9.00 (5.00, 14.00) | Z=0.880c | 0.379 |
| Survival time (days), M (Q1, Q3) | 1,095.00 (53.00, 1095.00) | 1,095.00 (52.00, 1095.00) | 1,095.00 (56.00, 1095.00) | Z=−0.063c | 0.950 |
| Survival status, n (%) | χ2=0.003b | 0.958 | |||
| Survival | 10,484 (62.50) | 7,340 (62.51) | 3,144 (62.47) | ||
| Death | 6291 (37.50) | 4,402 (37.49) | 1,889 (37.53) |
a, using t-test; b, using Chi-square; c, using Mann-Whitney. ICU, intensive care unit; CCU, coronary care unit; CSRU, cardiac surgery recovery unit; MICU, medical intensive care unit; SICU, surgical intensive care unit; TSICU, trauma surgical intensive care unit; FIO2, fraction of inspiration O2; HF, heart failure; CPD, chronic pulmonary disease; DM, diabetes mellitus; CHD, coronary heart disease; WBC, white blood cell; BUN, blood urea nitrogen; GCS, Glasgow Coma Scale; SOFA, Sequential Organ Failure Assessment.
Predictor selection and nomogram construction
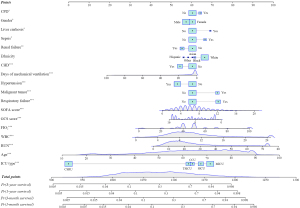
Age, gender, ethnicity, ICU type, comorbidity (HF, sepsis, CPD, hypertension, liver cirrhosis, DM, respiratory failure, malignant tumor, renal failure, and CHD), days of mechanical ventilation, WBC, BUN, FIO2, SOFA score, and GCS score were identified as significant predictors in the univariate Cox regression analysis for the training set (P<0.05) (Table 2). Furthermore, in the multivariate Cox stepwise regression, age, gender, ICU type, comorbidity (sepsis, CPD, hypertension, liver cirrhosis, respiratory failure, malignant tumor, and CHD), days of mechanical ventilation, WBC, BUN, FIO2, SOFA score, and GCS score were identified as significantly associated with the survival of ICU patients who received mechanical ventilation (P<0.05) (Table 3). Based on the predictors, the nomogram for predicting individuals’ short- and long-term survival was established (Figure 3).
Table 2
| Variables | HR (95% CI) | P |
|---|---|---|
| Age | 1.035 (1.033–1.037) | <0.001 |
| Gender | ||
| Female | Ref | |
| Male | 0.787 (0.741–0.835) | <0.001 |
| Ethnicity | ||
| Asian | Ref | |
| Black | 1.107 (0.894–1.369) | 0.351 |
| Hispanic/Latino | 0.634 (0.486–0.826) | <0.001 |
| Other | 0.721 (0.550–0.946) | 0.018 |
| White | 1.013 (0.839–1.222) | 0.894 |
| ICU type | ||
| MICU | Ref | |
| CSRU | 0.146 (0.132–0.162) | <0.001 |
| CCU | 0.850 (0.773–0.933) | <0.001 |
| SICU | 0.705 (0.651–0.763) | <0.001 |
| TSICU | 0.456 (0.414–0.502) | <0.001 |
| Days of mechanical ventilation | 1.030 (1.027–1.033) | <0.001 |
| HF | ||
| No | Ref | |
| Yes | 1.929 (1.816–2.048) | <0.001 |
| CPD | ||
| No | Ref | |
| Yes | 1.571 (1.458–1.693) | <0.001 |
| Hypertension | ||
| No | Ref | |
| Yes | 0.682 (0.642–0.725) | <0.001 |
| Sepsis | ||
| No | Ref | |
| Yes | 2.873(2.681–3.078) | <0.001 |
| Atrial fibrillation | ||
| No | Ref | |
| Yes | 1.341 (1.260–1.427) | <0.001 |
| Liver cirrhosis | ||
| No | Ref | |
| Yes | 1.898 (1.700–2.120) | <0.001 |
| DM | ||
| No | Ref | |
| Yes | 1.033 (0.961–1.111) | 0.374 |
| Respiratory failure | ||
| No | Ref | |
| Yes | 3.017 (2.843–3.202) | <0.001 |
| Malignant tumor | ||
| No | Ref | |
| Yes | 1.793 (1.680–1.914) | <0.001 |
| Renal failure | ||
| No | Ref | |
| Yes | 2.676 (2.517–2.844) | <0.001 |
| CHD | ||
| No | Ref | |
| Yes | 0.614 (0.574–0.657) | <0.001 |
| Laboratory indicators | ||
| FIO2 (%) | 1.017 (1.016–1.019) | <0.001 |
| WBC (k/μL) | 1.125 (1.118–1.131) | <0.001 |
| BUN (mg/dL) | 1.036 (1.035–1.038) | <0.001 |
| GCS score | 0.973 (0.967–0.979) | <0.001 |
| SOFA score | 1.144 (1.134–1.154) | <0.001 |
HR, hazard ratio; CI, confidence interval; ICU, intensive care unit; CCU, coronary care unit; CSRU, cardiac surgery recovery unit; MICU, medical intensive care unit; SICU, surgical intensive care unit; TSICU, trauma surgical intensive care unit; HF, heart failure; CPD, chronic pulmonary disease; DM, diabetes mellitus; CHD, coronary heart disease; FIO2, fraction of inspiration O2; WBC, white blood cell; BUN, blood urea nitrogen; GCS, Glasgow Coma Scale; SOFA, sequential organ failure assessment.
Table 3
| Variables | HR (95% CI) | P |
|---|---|---|
| Age | 1.029 (1.027–1.031) | <0.001 |
| Gender | ||
| Female | Ref | |
| Male | 0.928(0.872–0.986) | <0.001 |
| Ethnicity | ||
| Asian | Ref | |
| Black | 1.032 (0.833–1.279) | 0.771 |
| Hispanic/Latino | 0.901 (0.689–1.177) | 0.443 |
| Other | 0.953 (0.726–1.251) | 0.728 |
| White | 1.158 (0.959–1.398) | 0.129 |
| ICU type | ||
| MICU | Ref | |
| CSRU | 0.187 (0.166–0.211) | <0.001 |
| CCU | 0.812 (0.735–0.897) | <0.001 |
| SICU | 0.897 (0.823–0.978) | <0.001 |
| TSICU | 0.774 (0.697–0.858) | <0.001 |
| Days of mechanical ventilation | 0.990 (0.986–0.995) | <0.001 |
| CPD | ||
| No | Ref | |
| Yes | 1.571 (1.458–1.693) | <0.001 |
| Hypertension | ||
| No | Ref | |
| Yes | 0.682 (0.642–0.725) | <0.001 |
| Sepsis | ||
| No | Ref | |
| Yes | 2.873 (2.681–3.078) | <0.001 |
| Liver cirrhosis | ||
| No | Ref | |
| Yes | 1.898 (1.700–2.120) | <0.001 |
| Respiratory failure | ||
| No | Ref | |
| Yes | 3.017 (2.843–3.202) | <0.001 |
| Malignant tumor | ||
| No | Ref | |
| Yes | 1.793 (1.680–1.914) | <0.001 |
| Renal failure | ||
| No | Ref | |
| Yes | 2.676 (2.517–2.844) | <0.001 |
| CHD | ||
| No | Ref | |
| Yes | 0.614 (0.574–0.657) | <0.001 |
| FIO2 (%) | 1.017 (1.016–1.019) | <0.001 |
| WBC (k/μL) | 1.125 (1.118–1.131) | <0.001 |
| BUN (mg/dL) | 1.036 (1.035–1.038) | <0.001 |
| GCS score | 0.973 (0.967–0.979) | <0.001 |
| SOFA score | 1.144 (1.134–1.154) | <0.001 |
HR, hazard ratio; CI, confidence interval; ICU, intensive care unit; CCU, coronary care unit; CSRU, cardiac surgery recovery unit; MICU, medical intensive care unit; SICU, surgical intensive care unit; TSICU, trauma surgical intensive care unit; CPD, chronic pulmonary disease; CHD, coronary heart disease; FIO2, fraction of inspiration O2; WBC, white blood cell; BUN, blood urea nitrogen; GCS, Glasgow Coma Scale; SOFA, sequential organ failure assessment.
Predictive performance of the nomogram
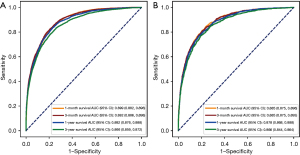
In the training set, the AUCs of the nomogram for 1-month, 3-month, 1-year, and 3-year survival prediction were 0.889 (95% CI: 0.882–0.896), 0.892 (95% CI: 0.886–0.898), 0.882 (95% CI: 0.876–0.888), and 0.866 (95% CI: 0.859–0.872), respectively. In the testing set, the AUCs of the nomogram for 1-month, 3-month, 1-year, and 3-year survival prediction were 0.885 (95% CI: 0.875–0.896), 0.885 (95% CI: 0.875–0.895), 0.878 (95% CI: 0.868–0.888), and 0.866 (95% CI: 0.844–0.864), respectively. The nomogram had a good predictive ability, with a C-index of 0.819 (95% CI: 0.813–0.825) and was validated in the testing set by a C-index of 0.816 (95% CI: 0.808–0.824) (Table 4 and Figure 4).
Table 4
| AUC (95% CI) | ||||
|---|---|---|---|---|
| 1-month survival | 3-month survival | 1-year survival | 3-year survival | |
| Training set | 0.889 (0.882–0.896) | 0.892 (0.886–0.898) | 0.882 (0.876–0.889) | 0.866 (0.859–0.873) |
| Testing set | 0.884 (0.873–0.895) | 0.884 (0.874–0.894) | 0.877 (0.867–0.887) | 0.866 (0.855–0.876) |
AUC, area under the curve; CI, confidence interval.
Validation of the predictive performance of the nomogram in different subgroups based on reasons for mechanical ventilation
For the subgroup of patients who received mechanical ventilation due to shock, the AUCs of the nomogram for 1-month, 3-month, 1-year, and 3-year survival prediction were 0.844 (95% CI: 0.829–0.860), 0.852 (95% CI: 0.837–0.868), 0.848 (95% CI: 0.832–0.863), and 0.844 (95% CI: 0.828–0.860), respectively. For the subgroup of patients who received mechanical ventilation due to sepsis, the AUCs of the nomogram for 1-month, 3-month, 1-year, and 3-year survival prediction were 0.829 (95% CI: 0.812–0.846), 0.834 (95% CI: 0.818–0.851), 0.830 (95% CI: 0.813–0.847), and 0.820 (95% CI: 0.803–0.838). For the subgroup of patients who received mechanical ventilation due to trauma, the AUCs of the nomogram for 1-month, 3-month, 1-year, and 3-year survival prediction were 0.865 (95% CI: 0.819–0.912), 0.882 (95% CI: 0.840–0.924), 0.873 (95% CI: 0.831–0.914), and 0.867 (95% CI: 0.824–0.909). For the subgroup of patients who received mechanical ventilation due to other causes, the AUCs of the nomogram for 1-month, 3-month, 1-year, and 3-year survival prediction were 0.883 (95% CI: 0.875–0.890), 0.881 (95% CI: 0.874–0.887), 0.870 (95% CI: 0.863–0.877), and 0.854 (95% CI: 0.847–0.860) (Table 5). The results of the subgroup validation indicated that our nomogram demonstrated good predictive performance for patients who received mechanical ventilation due to multiple causes, such as shock, sepsis, trauma, and other causes.
Table 5
| Models | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| Shock | |||||
| 1-month survival | 0.844 (0.829–0.860) | 0.756 (0.731–0.781) | 0.772 (0.749–0.796) | 0.765 (0.741–0.789) | 0.764 (0.739–0.789) |
| 3-month survival | 0.852 (0.837–0.868) | 0.755 (0.732–0.778) | 0.793 (0.768–0.819) | 0.835 (0.814–0.855) | 0.701 (0.674–0.728) |
| 1-year survival | 0.848 (0.832–0.863) | 0.718 (0.695–0.741) | 0.813 (0.787–0.839) | 0.868 (0.849–0.887) | 0.627 (0.598–0.655) |
| 3-year survival | 0.844 (0.828–0.860) | 0.829 (0.802–0.856) | 0.695 (0.672–0.717) | 0.893 (0.875–0.910) | 0.570 (0.541–0.599) |
| Sepsis | |||||
| 1-month survival | 0.829 (0.812–0.846) | 0.713 (0.686–0.740) | 0.799 (0.777–0.822) | 0.758 (0.732–0.785) | 0.759 (0.736–0.783) |
| 3-month survival | 0.834 (0.818–0.851) | 0.722 (0.698–0.746) | 0.794 (0.769–0.820) | 0.831 (0.810–0.853) | 0.670 (0.642–0.697) |
| 1-year survival | 0.830 (0.813–0.847) | 0.723 (0.700–0.746) | 0.779 (0.751–0.808) | 0.856 (0.836–0.875) | 0.608 (0.579–0.638) |
| 3-year survival | 0.820 (0.803–0.838) | 0.700 (0.677–0.723) | 0.793 (0.763–0.823) | 0.880 (0.862–0.898) | 0.550 (0.519–0.580) |
| Trauma | |||||
| 1-month survival | 0.865 (0.819–0.912) | 0.845 (0.768–0.923) | 0.752 (0.706–0.798) | 0.455 (0.377–0.533) | 0.952 (0.927–0.977) |
| 3-month survival | 0.882 (0.840–0.924) | 0.860 (0.790–0.931) | 0.772 (0.727–0.817) | 0.513 (0.434–0.591) | 0.952 (0.927–0.977) |
| 1-year survival | 0.873 (0.831–0.914) | 0.830 (0.756–0.904) | 0.777 (0.732–0.822) | 0.532 (0.454–0.610) | 0.937 (0.908–0.966) |
| 3-year survival | 0.867 (0.824–0.909) | 0.813 (0.739–0.887) | 0.784 (0.739–0.829) | 0.558 (0.480–0.636) | 0.926 (0.895–0.957) |
| Others | |||||
| 1-month survival | 0.883 (0.875–0.890) | 0.857 (0.842–0.872) | 0.749 (0.740–0.757) | 0.397 (0.383–0.412) | 0.964 (0.961–0.968) |
| 3-month survival | 0.881 (0.874–0.887) | 0.828 (0.814–0.843) | 0.774 (0.766–0.782) | 0.487 (0.473–0.501) | 0.946 (0.941–0.951) |
| 1-year survival | 0.870 (0.863–0.877) | 0.836 (0.823–0.848) | 0.752 (0.743–0.760) | 0.540 (0.526–0.553) | 0.929 (0.924–0.935) |
| 3-year survival | 0.854 (0.847–0.860) | 0.784 (0.771–0.797) | 0.772 (0.763–0.781) | 0.607 (0.594–0.620) | 0.888 (0.882–0.895) |
AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Example of the nomogram application
A 22.95-year-old male admitted to TSICU without sepsis, CPD, or hypertension had a white blood cell count of 11.1×109/µL, a SOFA score of 7, a GCS score of 9, FIO2 of 40%, and BUN of 18 mg/dL. According to our nomogram, the patient’s total score was 1030, and the predicted risk of death within 1 month, 3 months, 1 year, and 3 years was 0.0476, 0.0669, 0.0818, and 0.114, respectively. The patient’s actual situation was ‘survival,’ with a survival time ≥1,095 days, which indicated that the prediction from the nomogram was correct (Figure 5).

Discussion
In this study, we developed and validated a novel prediction tool for the survival of ICU patients who receive mechanical ventilation. The selected predictive factors included age, gender, ICU type, comorbidity (sepsis, CPD, hypertension, liver cirrhosis, respiratory failure, malignant tumor, and CHD), days of mechanical ventilation, WBC, BUN, FIO2, SOFA score, and GCS score. Based on those predictors, a predictive nomogram for survival in ICU patients receiving mechanical ventilation was established, with a C-index of 0.819, validated in the testing set by an index of 0.816. The AUCs of the nomogram for 1-month, 3-month, 1-year, and 3-year survival prediction were 0.889, 0.892, 0.882, and 0.866, respectively, and were validated in the testing set by indexes of 0.884, 0.884, 0.877, and 0.866, respectively. Additionally, subgroup validations based on the reasons for mechanical ventilation also showed good predictive performance of the nomogram for both short- and long-term survival.
The evidence is inconclusive regarding the association between age and survival in patients receiving mechanical ventilation. A previous study indicated that, despite the association between age and an increased weaning failure rate, age was not a significant predictor of prognosis in patients who received mechanical ventilation (16). Additionally, it was reported that patients with superior respiratory function and less comorbidity who received mechanical ventilation were more likely to experience a better prognosis, regardless of age (17). However, other studies have demonstrated a significant association between age and survival in patients who received mechanical ventilation. A multicenter cohort study conducted by Blot et al. found that older age was a risk factor for higher mortality in patients receiving mechanical ventilation (18). A Spanish study showed that older patients aged ≥75 years had increased ICU mortality compared with younger patients, with no difference in mechanical ventilation duration (19). Additionally, age was shown to be a predictor of mortality in patients who received mechanical ventilation in research conducted in Brazil, the USA, and China (20-22). Our results found that as age increased, the patient’s prognosis became worse, consistent with these studies (20-22). With increased age, organ reserve and compensatory function reduce, and the incidence of chronic disease increases, which might adversely impact survival. More attention should be paid to the safety of using mechanical ventilation in elderly ICU patients.
In the current research, higher GCS and lower SOFA scores were associated with a better prognosis in ICU patients who received mechanical ventilation. The GCS has good validity and reliability, and GCS scores have been shown to be correlated with mortality in ICU patients (23,24). SOFA scores have also been used to assess the prognosis of ICU patients (25). Previous research is consistent with our results and suggests that doctors should attempt to increase the GCS score and reduce the SOFA score in the ICU patient as much as possible before starting mechanical ventilation therapy. Future studies could investigate the best cutoff values for GCS and SOFA scores to optimize the management of ICU patients treated with mechanical ventilation.
Our results found that the existence of sepsis increased the risk of death in ICU patients who received mechanical ventilation. Sepsis, caused by a dysregulated host response to an infection, can result in life-threatening tissue damage and organ dysfunction (26,27). It can progress into septic shock, which involves circulatory dysfunction and abnormal cell metabolism, leading to substantially increased mortality (28), which might explain our result. Another study also suggested that sepsis might be a vital risk factor for mortality in elderly ICU patients who received mechanical ventilation (29). Those results indicated that ICU patients with sepsis who were treated with mechanical ventilation had a poor prognosis, and more effective adjuvant therapy should be investigated to improve their prognosis in future studies.
The present research showed that a higher serum creatinine level, an indicator of kidney function, was an independent predictor of decreased survival in ICU patients who received mechanical ventilation (30). Acute kidney injury has been associated with poor outcomes after discharge in patients admitted to the ICU (31). A previous study compared survival in patients with and without kidney damage who received mechanical ventilation and found that all patients who received renal replacement therapy died within 1 year (32). A meta-analysis demonstrated that renal dysfunction (both acute and chronic, regardless of the need for dialysis) was associated with 1-year mortality (33). Early assessment of the patient’s renal function and related treatment might improve the prognosis of ICU patients who receive mechanical ventilation. Additionally, we found that an increased number of WBCs was an independent predictor of survival in ICU patients who received mechanical ventilation. It has been reported that hospital-acquired infections are more likely to occur in ICU patients (34), and ICU-acquired infections have been shown to be independently associated with hospital mortality (35,36). The main reason for an increased WBC count is infection. Hence, reducing the risk of nosocomial infection might be an effective measure to improve the prognosis of ICU patients who receive mechanical ventilation.
Several models have been built to estimate survival in ICU patients who receive mechanical ventilation. Carson et al. developed a scoring model for predicting 1-year mortality in ICU patients requiring prolonged mechanical ventilation (37). In this model, points were assigned to age ≥65 years, age 50–64 years, platelets ≤150×109/L, vasopressors, and hemodialysis. However, long-term survival was not assessed, which is an essential element allowing clinicians and family members to conduct a comprehensive evaluation of patients. Our nomogram for predicting short- and long-term survival (1-month, 3-month, 1-year, and 3-year) in ICU patients who received mechanical ventilation was based on a large sample size, thereby providing a good foundation for the reliability of the prediction model. In addition, subgroup validations based on the reasons for mechanical ventilation (including shock, sepsis, trauma, and other causes) were conducted to reduce heterogeneity, and we found that the predictive performance of the nomogram was satisfactory in those four types of subgroups.
The nomogram developed in this study was based on a relatively large sample and had a moderate predictive ability. However, there are several limitations to this research. Firstly, one limitation of this study is the retrospective nature of the design. Secondly, the lack of external validation is another limitation of our study. Thirdly, more accurate indicators for mechanical ventilation could not be collected. Fourthly, since the data were extracted from a database, this may have limited the reasons for mechanical ventilation in this study. Our subgroup analysis based on shock, sepsis, trauma, and other causes may not be able to determine the nomogram’s predictive performance based on different mechanical ventilation causes. Further prospective multicenter studies are needed to validate the present model. In addition, many predictors were included in the current predicting model, future studies may attempt to use machine learning to simplify the model and make it more convenient for clinicians to apply it better.
Conclusions
Several predictors have been associated with the survival of ICU patients treated with mechanical ventilation, including age, gender, ICU type, comorbidity (sepsis, CPD, and hypertension), days of mechanical ventilation, WBC, BUN, FIO2, SOFA score, and GCS score. Based on those factors, a nomogram with good predictive performance for short- and long-term survival (1-month, 3-month, 1-year, and 3-year) in ICU patients treated with mechanical ventilation was developed and validated among 16,775 individuals from the MIMIC III database. We believe that the nomogram might provide a reference for physicians in clinical work to optimize the management of ICU patients who require mechanical ventilation based on individual short- and long-term survival.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-646/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-646/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pham T, Brochard LJ, Slutsky AS. Mechanical Ventilation: State of the Art. Mayo Clin Proc 2017;92:1382-400. [Crossref] [PubMed]
- Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med 2008;177:170-7. [Crossref] [PubMed]
- Su L, Zhang Z, Zheng F, et al. Five novel clinical phenotypes for critically ill patients with mechanical ventilation in intensive care units: a retrospective and multi database study. Respir Res 2020;21:325. [Crossref] [PubMed]
- Wunsch H, Wagner J, Herlim M, et al. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med 2013;41:2712-9. [Crossref] [PubMed]
- Urner M, Jüni P, Hansen B, et al. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med 2020;8:905-13. [Crossref] [PubMed]
- Udi J, Lang CN, Zotzmann V, et al. Incidence of Barotrauma in Patients With COVID-19 Pneumonia During Prolonged Invasive Mechanical Ventilation - A Case-Control Study. J Intensive Care Med 2021;36:477-83. [Crossref] [PubMed]
- Gattinoni L, Quintel M, Marini JJ. "Less is More" in mechanical ventilation. Intensive Care Med 2020;46:780-2. [Crossref] [PubMed]
- Machado-Alba JE, Usma-Valencia AF, Sánchez-Ramírez N, et al. Factors Associated with Survival in Patients Undergoing Invasive Mechanical Ventilation in an Intensive Care Unit in Colombia, 2017-2018: A Retrospective Cohort Study. Drugs Real World Outcomes 2021;8:417-25. [Crossref] [PubMed]
- Fialkow L, Farenzena M, Wawrzeniak IC, et al. Mechanical ventilation in patients in the intensive care unit of a general university hospital in southern Brazil: an epidemiological study. Clinics (Sao Paulo) 2016;71:144-51. [Crossref] [PubMed]
- Ferrando-Vivas P, Doidge J, Thomas K, et al. Prognostic Factors for 30-Day Mortality in Critically Ill Patients With Coronavirus Disease 2019: An Observational Cohort Study. Crit Care Med 2021;49:102-11. [Crossref] [PubMed]
- Lee SH, Kim MJ, Jeong ES, et al. Outcomes and prognostic factors in patients with prolonged acute mechanical ventilation: A single-center study in Korea. J Crit Care 2015;30:1016-20. [Crossref] [PubMed]
- Dai L, Yang D, Shen H. The power of clinical data empowered by clinical prediction model: an R tutorial. Ann Transl Med 2020;8:77. [Crossref] [PubMed]
- Zhou ZR, Wang WW, Li Y, et al. In-depth mining of clinical data: the construction of clinical prediction model with R. Ann Transl Med 2019;7:796. [Crossref] [PubMed]
- Hough CL, Caldwell ES, Cox CE, et al. Development and Validation of a Mortality Prediction Model for Patients Receiving 14 Days of Mechanical Ventilation. Crit Care Med 2015;43:2339-45. [Crossref] [PubMed]
- Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035. [Crossref] [PubMed]
- Dermot Frengley J, Sansone GR, Shakya K, et al. Prolonged mechanical ventilation in 540 seriously ill older adults: effects of increasing age on clinical outcomes and survival. J Am Geriatr Soc 2014;62:1-9. [Crossref] [PubMed]
- Huang C. How prolonged mechanical ventilation is a neglected disease in chest medicine: a study of prolonged mechanical ventilation based on 6 years of experience in Taiwan. Ther Adv Respir Dis 2019;13:1753466619878552. [Crossref] [PubMed]
- Blot S, Koulenti D, Dimopoulos G, et al. Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients*. Crit Care Med 2014;42:601-9. [Crossref] [PubMed]
- Añon JM, Gómez-Tello V, González-Higueras E, et al. Prognosis of elderly patients subjected to mechanical ventilation in the ICU. Med Intensiva 2013;37:149-55. [PubMed]
- Farfel JM, Franca SA, Sitta Mdo C, et al. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing 2009;38:515-20. [Crossref] [PubMed]
- George N, Moseley E, Eber R, et al. Deep learning to predict long-term mortality in patients requiring 7 days of mechanical ventilation. PLoS One 2021;16:e0253443. [Crossref] [PubMed]
- Ma JG, Zhu B, Jiang L, et al. Gender- and age-based differences in outcomes of mechanically ventilated ICU patients: a Chinese multicentre retrospective study. BMC Anesthesiol 2022;22:18. [Crossref] [PubMed]
- Hu C, Li L, Huang W, et al. Interpretable Machine Learning for Early Prediction of Prognosis in Sepsis: A Discovery and Validation Study. Infect Dis Ther 2022; [Epub ahead of print]. [Crossref] [PubMed]
- Wu Y, Huang S, Chang X. Understanding the complexity of sepsis mortality prediction via rule discovery and analysis: a pilot study. BMC Med Inform Decis Mak 2021;21:334. [Crossref] [PubMed]
- Hu T, Liu X, Liu Y. Usefulness of Glucose to Lymphocyte Ratio to Predict in-Hospital Mortality in Patients with AECOPD Admitted to the Intensive Care Unit. COPD 2022;19:158-65. [Crossref] [PubMed]
- Yang WS, Kang HD, Jung SK, et al. A mortality analysis of septic shock, vasoplegic shock and cryptic shock classified by the third international consensus definitions (Sepsis-3). Clin Respir J 2020;14:857-63. [Crossref] [PubMed]
- Fernando SM, Rochwerg B, Seely AJE. Clinical implications of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). CMAJ 2018;190:E1058-9. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Liang J, Li Z, Dong H, et al. Prognostic factors associated with mortality in mechanically ventilated patients in the intensive care unit: A single-center, retrospective cohort study of 905 patients. Medicine (Baltimore) 2019;98:e17592. [Crossref] [PubMed]
- Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 1992;38:1933-53. [Crossref] [PubMed]
- Barreto EF, Schreier DJ, May HP, et al. Incidence of Serum Creatinine Monitoring and Outpatient Visit Follow-Up among Acute Kidney Injury Survivors after Discharge: A Population-Based Cohort Study. Am J Nephrol 2021;52:817-26. [Crossref] [PubMed]
- Chao DC, Scheinhorn DJ, Stearn-Hassenpflug M. Impact of renal dysfunction on weaning from prolonged mechanical ventilation. Crit Care 1997;1:101-4. [Crossref] [PubMed]
- Dettmer MR, Damuth E, Zarbiv S, et al. Prognostic Factors for Long-Term Mortality in Critically Ill Patients Treated With Prolonged Mechanical Ventilation: A Systematic Review. Crit Care Med 2017;45:69-74. [Crossref] [PubMed]
- Gandra S, Ellison RT 3rd. Modern trends in infection control practices in intensive care units. J Intensive Care Med 2014;29:311-26. [Crossref] [PubMed]
- Grasselli G, Scaravilli V, Mangioni D, et al. Hospital-Acquired Infections in Critically Ill Patients With COVID-19. Chest 2021;160:454-65. [Crossref] [PubMed]
- Massart N, Mansour A, Ross JT, et al. Mortality due to hospital-acquired infection after cardiac surgery. J Thorac Cardiovasc Surg 2020; Epub ahead of print. [Crossref] [PubMed]
- Carson SS, Kahn JM, Hough CL, et al. A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation. Crit Care Med. 2012;40:1171-6. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)



