
Epigallocatechin gallate reduces uric acid levels by regulating xanthine oxidase activity and uric acid excretion in vitro and in vivo
Introduction
Recent improvements in living standards and dietary changes have led to a significant increase in the incidence of hyperuricemia, particularly in younger individuals (1). Hyperuricemia is the biochemical basis of gout and is closely related to hypertension, coronary heart disease, metabolic syndrome, and diabetes (2) and has become a public health problem worldwide. Uric acid-lowering drugs, such as allopurinol, febuxostat, benzbromarone, have not only good efficacy but also have several side effects (3). Therefore, it is important to identify new uric acid-lowering drugs with good efficacy and minimal side effects. In China, tea has been consumed for thousands of years; it is thought to reduce uric acid and improve gout. Tea polyphenols (TP) and their derivatives have been detected, including catechins, flavonoids, flower pigment, and phenolic acids; catechins account for 65–80% of the total TP. Catechin compounds in tea include catechins (C), epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), gallocatechin 3-O-gallate (GCG), and epigallocatechin gallate (EGCG), EGCG account for 50% of the total TP (4). TPs are linked to weight loss, the reduction of blood lipids, and anti-cancer, anti-osteoporosis, and other pharmacological activities (5). TP reduces the high uric acid levels in mouse serum induced by potassium; in particular, it reduces uric acid production and increases uric acid excretion (6). However, the effective component of TP in tea leaves involved in lowering uric acid is unclear. In this study, the effect of EGCG in tea leaves on uric acid was studied in vivo and in vitro to clarify the pharmacological effect of EGCG.
Methods
Chemicals and reagents
EGCG (Chengdu Purinol, China), oxonic acid potassium salt (Sigma-Aldrich Co. LLC, USA), DMEM (HyClone, Logan, UT, USA), CCK8 (Tongren, Japan), fetal bovine serum (BI, China), and allopurinol (Shanghai Xinyi, Shanghai, China) were obtained.
Cell culture
BRL 3A rat liver cells were provided by the Cell Bank of the Chinese Academy of Sciences, and cultured in DMEM supplemented with 10% fetal bovine serum under 5% CO2 at 37 °C.
Cell proliferation experiments
The effect of EGCG on BRL 3A cell proliferation was studied by the CCK8 method. The cell suspension was inoculated into a 96-well plate. After 24 h of culture, EGCG at different concentrations was added for 48 h. CCK8 reagent (10 µL) was added to each well and incubated in an incubator for 1 h. The absorbance was measured at 450 nm (SpectraMax I3 Austria). The inhibition rate was calculated according to the formula: inhibition rate (%) = (1– absorbance of treatment group/control group) ×100%.
Determination of uric acid and xanthine oxidase (XOD)
Uric acid and XOD were measured using uric acid and XOD test kits (Nanjing Jiancheng). After homogenization, the tissue protein concentration of XOD was measured, and the level of XOD activity in tissues was calculated to follow by the procedure manual.
Animal experiments
Sixty male Sprague-Dawley rats were purchased from Hunan Slaike Jingda Experimental Animals Co., LTD. (certificate No. SCXK 2016-0002). A chronic hyperuricemia model was established by the subcutaneous injection of oxonic acid potassium salt (200 mg/kg) and the intragastric administration of ethambutol (250 mg/kg) once a day for 7 days. The rats were randomly divided into a normal group, model group, high-, medium-, and low-dose EGCG groups (100, 50, and 25 mg/kg, respectively), and an allopurinol group (10 mg/kg). One hour after the model was established, the drug was given by gavage once a day for 7 days. Blood was collected from the eye socket 3 h after the last model was made, and the liver and kidney were preserved after the animals were killed.
Determination of serum uric acid and XOD
The rat liver was added to normal saline to make a 10% tissue homogenate, and the supernatant was centrifuged to determine the concentration of XOD.
RT-qPCR
Total RNA from the renal tissue was extracted using TRIzol reagent, followed by cDNA synthesis by the Revert Aid First Strand cDNA Synthesis Kit (Eppendorf Mastercycler). The primers are shown in Table 1. The conditions were: predenaturation at 95 °C for 1 min, followed by 40 cycles of 95 °C for 3 s and 60 °C for 20 s. The mRNA quantity was calculated based on cycle threshold (CT) values by Applied Biosystems 7500 v.2.0.6. The relative mRNA expression levels were determined by the 2–ΔΔCt method.

Full table
Immunohistochemical staining
The kidney tissues were fixed in 10% neutral formalin solution. Ethanol gradient dehydration, xylene transparency, wax immersion, embedding, and slicing at a thickness of 5 µm were performed. Immunohistochemical staining was performed by the SP method, and the primary antibody was replaced with phosphate-buffered saline for the negative control. DAB and hematoxylin staining were performed. Rats were randomly selected from each group, sections were made from each kidney, and high-power (×200) visual fields were evaluated using Image-pro plus 6.0.
Statistical treatment
The data are expressed as . SPSS17.0 was used for statistical analyses. One-way ANOVA was used for comparisons among groups. P<0.05 was considered statistically significant.
Results
Effect of EGCG on survival in BRL 3A hepatocytes
After 24 h of culture in 24-well plates, EGCG (20, 40, 60, 80, and 100 µL) was added successively for 48 h. Cell activity was determined by CCK8 assays. The results showed that EGCG had no significant effect on the growth and proliferation of hepatocytes in the 0, 20, 40, 60, 80, and 100 µM dose groups, suggesting that EGCG is safe, as shown in Figure 1.
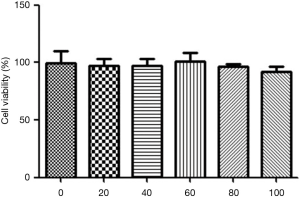
Effect of EGCG on uric acid levels and XOD activity in rat BRL 3A hepatocytes
We evaluated the effects of EGCG on uric acid in vitro. In xanthine-pretreated cell supernatants, XOD activity and uric acid were significantly higher than those in control cells. EGCG at 40 and 10 µL significantly inhibited XOD activity (P<0.05) and reduced the levels of uric acid (P<0.05), as shown in Figure 2.
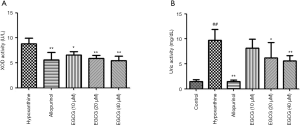
Effects of EGCG on XOD and serum uric acid levels in hyperuricemia rats
XOD levels were significantly higher in the serum and liver tissues in the group with high uric acid than in the normal group (P<0.01). Compared with the group with high uric acid, XOD levels in the serum and liver tissues in the group treated with EGCG (50 and 100 mg/kg) were significantly lower (P<0.05). Serum uric acid levels in the allopurinol group and the EGCG (50 and 100 mg/kg) groups were significantly lower than those in the model group (P<0.01), indicating that EGCG reduces uric acid in hyperuricemia rats, as shown in Figure 3.
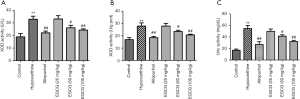
Effect of EGCG on mOAT1 and mGLUT9 expression in renal tissues of hyperuricemia rats
Compared to the normal controls, the levels of mGLUT9 were significantly higher in hyperuricemia rats. mOAT1 levels were significantly lower (P<0.05) in hyperuricemia rats than in normal controls. The EGCG group showed significantly higher levels of mOAT1 than those in hyperuricemia rats, and all EGCG groups showed significantly decreased expression of mGLUT9 (Figure 4).
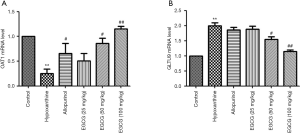
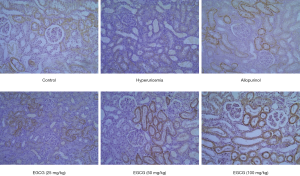
Effect of EGCG on OAT1 expression in renal tissues of hyperuricemia rats
OAT1 was expressed in the kidney tissues of rats in the control group. Compared with the normal group, OAT1 expression in the kidney tissues of rats in the model group was significantly lower. Compared with the model group, OAT1 levels in rats in the 50 and 100 mg/kg EGCG groups and allopurinol groups were significantly higher (P<0.05), as shown in Figure 5.
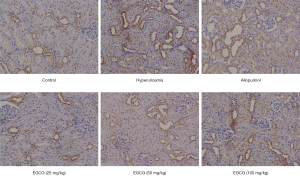
Effect of EGCG on GLUT9 expression in renal tissues of hyperuricemia rats
The expression of GLUT9 in the kidney of rats in the normal group was weakly positive. GLUT9 expression in the kidney of rats was significantly higher in the model group than in the control group (P<0.01), as shown in Figure 6.
Discussion
Disorders of uric acid metabolism can be due to excessive production or insufficient excretion of uric acid in the blood. The main causes of hyperuricemia are purine metabolism disorders and urate excretion reductions (7,8). Hyperuricemia can lead to many diseases, such as gout, arthritis, kidney injury, fat metabolism disorders, and diabetes (9-12).
Oxonic acid potassium salt can inhibit uric acid decomposition, increase uric acid levels in the body, and is commonly used to establish animal models. Ethambutol competitively inhibits the secretion of uric acid from the kidney, and the dual inhibition of uric acid excretion from the kidneys reduces blood uric acid levels in the body. In this study, oxonic acid potassium salt combined with ethambutol was used to replicate the hyperuricemia model in rats successfully, and the serum uric acid level increased steadily, as expected.
The liver is the main site for the production of uric acid, and XOD is the key rate-limiting enzyme for the production of uric acid in the body. Hypoxanthine generates uric acid by the two-step oxidation of XOD (13). An increase in liver XOD activity can increase the production of uric acid in vivo, and anti-gout and uric acid-lowering drugs have mainly been aimed at the inhibition of XOD activity (14). In this study, rat BRL 3A liver cells were cultured in vitro, and EGCG intervention after xanthine stimulation significantly inhibited cell XOD activity and reduced uric acid levels. In vivo, EGCG significantly reduced the uric acid level and inhibited XOD activity in the serum and liver tissues of hyperuricemia rats.
Kidney uric acid excretion mainly involves glomerular filtration, renal tubules, and collecting duct absorption, and re-secretion after reabsorption. About 98% of glomerular uric acid is absorbed by renal tubules (15). OAT1 is encoded by SLC22A6, located on chromosome 11q13.1, consisting of 10 exons and 9 introns. Eraly et al. (16) found that in OAT1 knockout mice, the ability of renal tubules to secrete urate was significantly reduced, and human organic anion transporter 1 (hOAT1) was the main substance regulating renal uric acid secretion. hOAT1 plays an important role in the absorption of urate from the portal vein space to renal tubular epithelial cells. It has time-dependent and dose-dependent effects on the transport of uric acid. Many organic and inorganic anions can affect the transport of uric acid via hOAT1. GLUT9, also known as a voltage-driven urate transporter 1 (URATv1), belongs to the glucose transporter family and is a high flux and low energy urate transporter (17). Glut9, encoded by SLC2A9, is divided into two subtypes, Glut9a and Glut9b. Glut9a is expressed in several metabolic organs, while Glut9b is found only in the liver and kidney. The renal tubule is the main site of Glut9 expression. Therefore, Glut9 is a risk factor for hyperuricemia. Vitart et al. (18) studied a Croatian genome and found that SLC2A9 explained 1.7–5.3% of the variation in the uric acid concentration. The contribution differed between men and women (17) (i.e., 5–6% for women and 1–2% for men). Increasing research has focused on the development of glut9-related drugs (19). In this study, EGCG significantly promoted the expression of OAT1 and inhibited the expression of GLUT9 in renal tissues of hyperuricemia rats.
EGCG can significantly reduce uric acid levels in xanthine-stimulated BRL 3A liver cells and can significantly reduce uric acid levels in oxygen oxazine and potassium combined with ethambutol-induced hyperuricemia rats. Its mechanism of action may be related to the inhibition of the activity of the uric acid synthetase XOD activity, the promotion of OAT1, and the inhibition of GLUT9. The results reveal the basis for understanding high uric acid-related hematic disease resistance and demonstrate the potential clinical applications of EGCG.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Experimental Animal Ethics Committee of Jiangxi University of Traditional Chinese Medicine (No. JZLLSC2018-0094).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rho YH, Zhu Y, Choi HK. The epidemiology of uric acid and fructose. Semin Nephrol 2011;31:410-9. [Crossref] [PubMed]
- Kuwabara M. Hyperuricemia, Cardiovascular Disease, and Hypertension. Pulse (Basel) 2016;3:242-52. [Crossref] [PubMed]
- Castrejon I, Toledano E, Rosario MP, et al. Safety of allopurinol compared with other urate-lowering drugs in patients with gout: a systematic review and meta-analysis. Rheumatol Int 2015;35:1127-37. [Crossref] [PubMed]
- Wang Y, Ho CT. Polyphenolic chemistry of tea and coffee: a century of progress. J Agric Food Chem 2009;57:8109-14. [Crossref] [PubMed]
- Chen ZM, Lin Z. Tea and human health: biomedical functions of tea active components and current issues. J Zhejiang Univ Sci B 2015;16:87-102. [Crossref] [PubMed]
- Chen G, Tan ML. Effect of green tea polyphenols on uric acid level in potassium oxonate-induced hyperuricemic mice and mechanism. Chinese Pharmacological Bulletin 2017;33:218-22.
- Hu QH, Jiao RQ, Wang X, et al. Simiao pill ameliorates urate underexcretion and renal dysfunction in hyperuricemic mice. J Ethnopharmacol 2010;128:685-92. [Crossref] [PubMed]
- Wang LY, Wang CJ, Wang MZ, et al. Study of Protection of Aqueous Extract of Arctium lappa Root on Hyperuricemia Mice. China Pharmacy 2011;22:4054-5.
- Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care 2009;32:1737-42. [Crossref] [PubMed]
- Kanungo S, Wells K, Tribett T, et al. Glycogen metabolism and glycogen storage disorders. Ann Transl Med 2018;6:474. [Crossref] [PubMed]
- Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 2007;120:442-7. [Crossref] [PubMed]
- Gazzaruso C, Coppola A, Montalcini T, et al. Anti-diabetic agents and heart health: how to use new diabetes medications in a global strategy for the prevention of cardiovascular complications in type 2 diabetes. Ann Transl Med 2018;6:195. [Crossref] [PubMed]
- Kong LD, Cai Y, Huang WW, et al. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol 2000;73:199-207. [Crossref] [PubMed]
- Wang X, Wang CP, Hu QH, et al. The dual actions of Sanmiao wan as a hypouricemic agent: down-regulation of hepatic XOD and renal mURAT1 in hyperuricemic mice. J Ethnopharmacol 2010;128:107-15. [Crossref] [PubMed]
- Lipkowitz MS. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep 2012;14:179-88. [Crossref] [PubMed]
- Eraly SA, Vallon V, Rieg T, et al. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics 2008;33:180-92. [Crossref] [PubMed]
- Wright AF, Rudan I, Hastie ND, et al. A 'complexity' of urate transporters. Kidney Int 2010;78:446-52. [Crossref] [PubMed]
- Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 2008;40:437-42. [Crossref] [PubMed]
- Lipkowitz MS, Leal-Pinto E, Cohen BE, et al. Galectin 9 is the sugar-regulated urate transporter/channel UAT. Glycoconj J 2002;19:491-8. [Crossref] [PubMed]


