
Proprioceptive changes measured by histopathological and electrophysiological evaluations after NGF injection of anterior cruciate ligament reconstruction
Introduction
An anterior cruciate ligament (ACL) is one of the major ligaments that, with resistance to anterior tibia deformation and rotational load (1), and its injury could result in balance disorder and proprioceptive dysfunction (2,3). Proprioception has been reported to be composed of incoming and outgoing pathways of somatosensory systems that control the reflexes and muscle tone of muscles contributing to regulating the preciseness of the articular angles of the knee joint (4). Consequently, proprioceptive dysfunction should also be assessed after ACL reconstruction surgery. Currently, there are few treatment methods, including reconstructing ACL and rehabilitation exercise to reconstruct the proprioception, applied to the reconstruction of ACL injury proprioception (1,5). However, several disadvantages have been presented, suggesting that ACL reconstruction or several months of rehabilitation might not result in improvement proprioception due to limitations such as the lack of ACL residue or uncertainty of efficacy along with long recovery time (6).
Nerve growth factor (NGF) is a neurotrophic factor released by mast cells, lymphocytes, and monocytes/macrophages in response to tissue inflammation and nociception (7). As a small secretory protein, NGF has been reported to play important roles in nerve cell proliferation and function (8). Moreover, NGF engages in the differentiation and survival of specific target neurons contributing to the maintenance and development of the sensory nervous system (9,10). According to the principle of osseoperception, it may be effective for promoting proprioception of the dental implant to induce peri-implant nerve regeneration, and He et al. (11) has revealed that NGF stimulates nerve regeneration and bone formation by its biological activities on both neuronal and non-neuronal cells. But until now, the effect of NGF on proprioceptive function after ACL reconstruction has not been reported.
In this study, we examined the role of NGF in the proprioceptive system following ACL injury rehabilitation, changes in the somatosensory evoked potentials (SEPs) and electromyograms (EMG), as well as the morphology and quantities of proprioceptors in white New Zealand rabbits after ACL injury injection.
Methods
Study design and research equipment
A total of 28 mature New Zealand white rabbits, weighing 1,500–2,500 g, were used in this study. The rabbits were randomized into 3 groups: the blank control group (n=4), the experimental group (n=12), and the experimental control group (n=12). The Laboratory Animal Center approved this study of the Gansu University of Chinese Medicine, and animal care was following the “Guide for the Care and Use of Laboratory Animals”.
The research equipment were as following: rotary type slicer (RM2135 type, LEICA company), optical microscope (BH2, push around), induce voltmeter (photoelectric MEB2200 type, Japan), and muscle all (photoelectric MEB2200 type, Japan), electronic balance (AR1530/C, OHAUS companies in the United States), 81-2 type constant temperature magnetic stirrer (Shanghai music instrument factory), PHS-3 ph meter (Shanghai Yi instrument factory), portable pressure steam sterilizer (Jiangyin Binjiang medical equipment factory), automatic double distilled water machine (Shanghai Broadcom), electrothermal blowing (Chongqing Sida experimental apparatus), ultra-low temperature freezer (ULT1386-3 v, the GS), BH-type 2 biological microscope.
Surgical technique
The treatment of the three groups is briefly summarized in Table 1. Specifically, rabbits were anesthetized using 3% pentobarbital (1 mL/kg). After proper anesthesia, the rabbits were fixed in the supine position with shaved skin on the surgical area and sterilized. First, the operators marked the skin incision in the center of the knee and the iliac medial side and removed the fascia from the muscle. Next, it was washed with 30°C sterile normal saline, 3/4 of the ACL was cut off, leaving 1/4 of the ACL connecting to the femur and tibia. The wound was closed, disinfected, and covered with penicillin powder. Three days after the operation, 1×104 penicillin injection (1 time/24 h, IV) was used to prevent infection.

Full table
The anterior tibial tendon and periosteum of the medial tibia (length ~1.5 cm, diameter ~0.5 cm) were excised and used as the tendon graft. Next, the tendon was woven to 3 cm with the non-absorbable line 1, and the femoral head was 3 mm in diameter and 4 mm in the tibia. The graft was pre-tensioned manually with 20 N and was then fixed with the doornail and non-absorbable line 1 outside of the femoral tunnel and tibial tunnel. The wound was cleaned, sutured and pressed by the elastic bandage. All surgery was performed under general anesthesia, as well as all the operations were to minimize the suffering during the operation. After the 2nd month of the operation, NGF (20 µg/week; N2513 Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was injected into the bilateral knee joint of the rabbits in the experimental group.
Histopathological evaluation
At the 2nd, 4th, and 6th months of ACL reconstruction, 4 rabbits were randomly obtained from the experimental group and control group, respectively. SEPs and EMG were performed for electro neurophysiological detection. Accordingly, the rabbits were executed by air embolism, and then the ligaments of the rabbits were removed and stained with gold chloride.
Electrophysiological evaluation
Under inhaling ether and local anesthesia in 1% lidocaine, the head and limbs of rabbits were fixed. After exposing the ACL, the electrodes were placed between the posterior margin of the two orbiters and the median sagittal line at 0.5 and 0.2 cm by the side, which was the sensory area of the lateral posterior extremity. Moreover, the reference electrode was placed under the subcutaneous of the nasal root, and the proximal end of the lower limb was grounded on the electrode. SEPs were measured at 26–28 °C, and the stimulation was constant at an intensity of 18–20 V with a single square wave (the wave width was 0.1 ms). Next, SEPs were recorded using the photoelectric evoked potentiometer, and the information was input to the microcomputer operating system.
Biostatistical evaluation
For EMG examination, the electrode was placed in the hamstring muscle, and the reference electrode was placed in malleolus medialis. The bipolar surface electrode was placed at the ACL adherent at 26–28 °C. The stimulation parameters were constant with the wave width at 0.1 ms and the frequency at 3 Hz. Next, EMG was monitored and recorded using the photoelectric electromyography, and the waveform and latency of EMG were analyzed.
Gold chloride staining
Firstly, the ACLs of all rabbits were obtained completely and soaked in fresh lemon juice and 88% acid mixture at room temperature for 15 min. Secondly, ACLs were placed in l% gold chloride solutions for 30 min, and then 25% formic acid solution was added for 15 h. After washed by the distilled water for 1 h, the ACLs were put in the pure glycerin for 24 h in order. Next, tissue blocks were dehydrated with alcohol gradient, cleared in xylene, embedded in paraffin, and filleted at the thickness of 15 µm. For avoiding repetition, the femoral head, tibia end, and middle part of the specimens were marked and two operators respectively counted the number of proprioceptors.
Statistical analysis
All data were presented as mean ± SD, and the statistical analysis was performed by SPSS 13.0 software. One-way ANOVA was used to compare groups, and the normal test was performed using the Kolmogorov-Smirnov (K-S) test. A P<0.05 was statistically significant.
Results
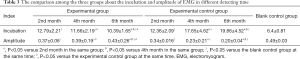
Compared with the blank control group, the incubations of SEPs and EMG were both extended in the experimental group and the experimental control group at 2nd, 4th, and 6th months, respectively. Moreover, incubations of SEPs and EMG in the experimental group at 4th and 6th months were significantly decreased when compared to those in the experimental control group (all, P<0.05; Tables 2,3 and Figure 1A,B), suggesting NGF could effectively shorten the incubation. However, the difference of incubation between the experimental group and the blank control group was still obvious at 6th month (P<0.05). On the contrary, the amplitudes of SEPs and EMG in the experimental group and the experimental control group were decreased compared with the blank control group at the same time point, respectively. And the amplitudes in experimental group were significantly higher than those in experimental control group at 4th and 6th months, respectively (all, P<0.05; Tables 2,3 and Figure 1C,D), but the amplitudes of experimental group were still lower than those in the blank control group at 6th month (P<0.05). The results revealed that NGF could effectively put off the decline of proprioceptor function altogether.

Full table
Full table
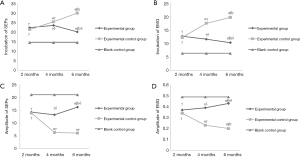
To further prove the role of NGF on the proprioception function after ACL reconstruction surgery, the number of proprioceptors was recorded after gold chloride staining. As shown in Figure 2, the volume of the Ruffini corpuscle and Pacinian corpuscle in the experimental control group were reduced, the leaf shape gradually disappeared, as well as the thickness of the packaging plate was smaller over time. As for the experimental group, there was a leaf structure found in the Ruffini corpuscle, and the atypical structure was reduced over time.
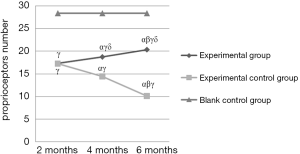
Furthermore, the number of receptors in the experimental control group significantly decreased over time (P<0.05), but in the experimental group was remarkably up-regulated (P<0.05) (Table 4 and Figure 3). More importantly, in comparison with the experimental control group, the number of receptors in the experimental group was increased at both 4th and 6th months, and the changes were significant (both P<0.05), suggesting the injection of NGF could promote the generation of proprioceptors. However, the difference of proprioceptors number between the experimental group and the blank control group was significant (P<0.05).

Full table
Discussion
The findings in the present study showed that ACL reconstruction without rehabilitation training was limited to the recovery of proprioceptor function, and NGF had a significant effect on proprioceptor function and quantity after ACL reconstruction.
During the past few years, the research on the treatment of proprioceptive rehabilitation is based on physical rehabilitation, but the curative effect is not satisfactory. Furthermore, instead of the biomechanical stability of the restorative joints, the research of proprioception in supporting the dynamic stability of the knee joint has received increased attention. Proprioception has been reported to play a significant role in the regulation of the nervous system and motor performance (12), and it has a significant correlation with the natural feeling of the knee joint (13,14). Since Freeman et al. (15) found the improved chlorinated gold staining method was more convenient, clear and stable for research on the distribution of nerve in 1967, proprioception has been defined as an important parameter for functional rehabilitation of knee joint because of its correlation with knee function (16,17).
Currently, SEPs and EMG have been recognized as the methods for examining the sensory state of the animal’s knee. For example, Lu et al. (18) revealed that the incubation of hamstring contraction related to the functional instability of the knee joint is often significantly prolonged after ACL injury, which can be used as an indicator of proprioceptive loss after the ACL injury. In the present study with the rabbit model, the electrophysiological test showed that compared with the blank control group, the incubation of SEPs and EMG in both the experimental group and the experimental control group were prolonged, and the amplitude decreased. The result indicated the ACL reconstruction without rehabilitation training could not significantly improve the reduction and function of the proprioceptor, which was following the previous study (19-21). Moreover, in comparison to the experimental control group, the experimental group injected with NGF performed shorter incubation and higher amplitude, suggesting NGF could promote the improvement of proprioception function after ACL reconstruction. However, the statistically significant differences of incubation and amplitude were noted between the experimental group and blank control group at the 6th month, which was speculated to due to the dose and use time of NGF. Hence, further research to investigate whether NGF could help proprioception of ACL to return to the normal level is still needed.
As a potential neurotrophic factor that may be used to reduce lidocaine-induced neurotoxicity, NGF may promote the growth and regeneration of neurons (22). Svensson et al. (23) revealed that the masseter muscle injection of NGF is associated with a distinct and prolongs sensitization to mechanical stimuli. Moreover, an increasing number of studies suggest that gold chloride staining has played a significant role in the observation of the existence, morphology, and distribution of proprioceptors (24). In our study, the results showed that the number of receptors in the experimental group injected with NGF was significantly increased, and the atypical structure was reduced when compared with the experimental control group, suggesting NGF plays an important role in improving the proprioception function after ACL reconstruction. However, it was previously demonstrated that the numbers of mechanoreceptors significantly decreased, and their morphological changed appear with aging in rabbits (25); therefore, further investigation about the effect of NGF on the proprioception function after ACL reconstruction based on different age groups of rabbits still needed.
Conclusions
In summary, our study has proved that the injection of NGF could improve joint function rehabilitation by promoting function and quantity of proprioception after ACL reconstruction. However, to investigate whether NGF could restore the function and quantity of the proprioceptor of ACL reconstruction to the normal state and further study still is needed.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Laboratory Animal Center approved this study of the Gansu University of Chinese Medicine, and animal care was following the “Guide for the Care and Use of Laboratory Animals”.
References
- Cho SH, Bae CH, Gak HB. Effects of closed kinetic chain exercises on proprioception and functional scores of the knee after anterior cruciate ligament reconstruction. J Phys Ther Sci 2013;25:1239-41. [Crossref] [PubMed]
- Delahunt E, Chawke M, Kelleher J, et al. Lower limb kinematics and dynamic postural stability in anterior cruciate ligament-reconstructed female athletes. J Athl Train 2013;48:172-85. [Crossref] [PubMed]
- Fox JA, Pierce M, Bojchuk J, et al. Revision anterior cruciate ligament reconstruction with nonirradiated fresh-frozen patellar tendon allograft. Arthroscopy 2004;20:787-94. [Crossref] [PubMed]
- Porter RE. Motor Control: Theory and Practical Applications, 2d ed. Physiotherapy 1996;82:591.
- Dhillon MS, Bali K, Prabhakar S. Proprioception in anterior cruciate ligament deficient knees and its relevance in anterior cruciate ligament reconstruction. Indian J Orthop 2011;45:294-300. [Crossref] [PubMed]
- Lee DH, Lee JH, Ahn SE, et al. Effect of Time after Anterior Cruciate Ligament Tears on Proprioception and Postural Stability. PLoS One 2015;10:e0139038. [Crossref] [PubMed]
- Kennedy AE, Laamanen CA, Ross MS, et al. Nerve growth factor inhibitor with novel-binding domain demonstrates nanomolar efficacy in both cell-based and cell-free assay systems. Pharmacol Res Perspect 2017;5:e00339. [Crossref] [PubMed]
- Theveneau E, Marchant L, Kuriyama S, et al. Collective chemotaxis requires contact-dependent cell polarity. Developmental Cell 2010;19:39. [Crossref] [PubMed]
- Bilici A, Ustaalioglu BB, Seker M, et al. Clinical value of FDG PET/CT in the diagnosis of suspected recurrent ovarian cancer: is there an impact of FDG PET/CT on patient management. Eur J Nucl Med Mol Imaging 2010;37:1259-69. [Crossref] [PubMed]
- Miyagi M, Ishikawa T, Kamoda H, et al. Efficacy of nerve growth factor antibody in a knee osteoarthritis pain model in mice. BMC Musculoskelet Disord 2017;18:428. [Crossref] [PubMed]
- He H, Yao Y, Wang Y, et al. A novel bionic design of dental implant for promoting its long-term success using nerve growth factor (NGF): Utilizing nano-springs to construct a stress-cushioning structure inside the implant. Med Sci Monit 2012;18:HY42-6. [Crossref] [PubMed]
- Barrack RL, Lund PJ, Skinner HB. Knee joint proprioception revisited. Journal of Sport Rehabilitation 2010;3:18-42. [Crossref]
- Eggli S, Röder C, Perler G, et al. Five year results of the first ten ACL patients treated with dynamic intraligamentary stabilisation. BMC Musculoskelet Disord 2016;17:105. [Crossref] [PubMed]
- Roberts D, Andersson G, Fridén T. Knee joint proprioception in ACL-deficient knees is related to cartilage injury, laxity and age: a retrospective study of 54 patients. Acta Orthopaedica Scandinavica 2004;75:78. [Crossref] [PubMed]
- Freeman MA, Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J Anat 1967;101:505-32. [PubMed]
- Filardo G, Roffi A, Merli G, et al. Patient kinesiophobia affects both recovery time and final outcome after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2016;24:3322-8. [Crossref] [PubMed]
- Borda IM, Ungur R, Irsay L, et al. Benefits of a postoperative rehabilitation program on strength recovery after total knee arthroplasty. Palestrica of the Third Millennium Civilization & Sport 2014;15:31.
- Lu HH, Jr CJ, Manuel S, et al. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials 2005;26:4805. [Crossref] [PubMed]
- Kim SH, Jung YB, Song MK, et al. Comparison of double-bundle anterior cruciate ligament (ACL) reconstruction and single-bundle reconstruction with remnant pull-out suture. Knee Surg Sports Traumatol Arthrosc 2014;22:2085-93. [Crossref] [PubMed]
- Kim HS, Seon JK, Jo AR. Current trends in anterior cruciate ligament reconstruction. Knee Surgery & Related Research 2013;25:165. [Crossref] [PubMed]
- Reider B, Arcand MA, Diehl LH, et al. Proprioception of the knee before and after anterior cruciate ligament reconstruction. Arthroscopy 2003;19:2-12. [Crossref] [PubMed]
- Manni L, Rocco ML, Bianchi P, et al. Nerve growth factor: basic studies and possible therapeutic applications. Growth Factors 2013;31:115-22. [Crossref] [PubMed]
- Svensson P, Wang K, Arendt-Nielsen L, et al. Effects of NGF-induced muscle sensitization on proprioception and nociception. Exp Brain Res 2008;189:1-10. [Crossref] [PubMed]
- Witherspoon JW, Smirnova IV, Mciff TE. Improved gold chloride staining method for anatomical analysis of sensory nerve endings in the shoulder capsule and labrum as examples of loose and dense fibrous tissues. Biotech Histochem 2014;89:355. [Crossref] [PubMed]
- Aydoğ ST, Korkusuz P, Doral MN, et al. Decrease in the numbers of mechanoreceptors in rabbit ACL: the effects of ageing. Knee Surg Sports Traumatol Arthrosc 2006;14:325-9. [Crossref] [PubMed]


