5-HT3 receptor antagonists for the treatment of nausea/vomiting
Abstract: It is well appreciated that a number of things (e.g., various insults, chemotherapeutic agents, radiation) may lead to the release of serotonin from the enterochromaffin of the gastrointestinal tract. Released serotonin may then bind to certain 5-HT3 receptors and promote nausea/vomiting. 5-HT3 receptor antagonists may ameliorate nausea/vomiting in a number of circumstances and have been utilized as important antiemetics for multiple conditions such as chemotherapy-induced nausea/vomiting (CINV), radiation-induced emesis (RIS), and postoperative nausea/vomiting (PONV).
Key words: Nausea/Vomiting; 5-HT3 antagonists; serotonin; chemotherapy-induced nausea/vomiting; postoperative nausea/vomiting; palonostron; ondansetron
Introduction
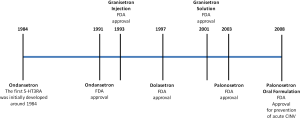
In 1957, J. H Gaddum and Zuleika P. Picarelli at the University of Edinburgh, discovered two serotonin receptor subtypes, the M and D receptors. The function of the M and D receptors could be blocked by morphine and dibenzyline, respectively. Although Gaddum and Picarelli may not have known at the time, this was the beginning of the discovery of the 5-HT3 receptor antagonists or serotonin antagonists. The 5-HT3 receptor was later found to correspond to the M receptor. In the 1970’s, John Fozard proved that metoclopramide and cocaine were weak antagonists at the 5-HT3 receptor. Fozard and Maurice Gittos eventually synthesized the first truly potent and selective 5-HT3 receptor antagonist (5-HT3RA), ondansetron. In the early 1990’s the first selective 5HT3 receptor antagonists were developed, ondansetron and granisetron. Tropisetron and dolasetron were developed in 1994 and 1997, respectively, followed by the newer second generation 5-HT3 receptor antagonist, palonosetron in 2003. Figure 1 reveals a timeline of the FDA approved 5-HTRAs (Figure 1).
The development of the selective 5HT3 receptor antagonists dramatically improved the treatment of nausea and vomiting. The selective 5HT3 receptor antagonists are the cornerstone of antiemetic therapy for patients receiving chemotherapy agents providing moderate to high antiemetic potential.
Serotonin, 5-HT, is found throughout the gut and central nervous system. In the gut, 5-HT is found in the mucosal enterochromaffin cells which are the sensory transducers which release 5-HT to activate intrinsic (via 5-HT4 and 5HT1P receptors) and extrinsic (via 5HT3 receptors) primary afferent nerves.
Approximately 80% of total body serotonin is found in the GI tract, the remainder being divided between the platelets, which avidly take up free serotonin, and the central nervous system. Ninety five percent of GI 5-HT is found within the granules of the enteroendocrine cells (ECs) secretory located mainly at the base of the crypts (1).
Factors which may lead to 5-HT exocytosis include: mechanical stimuli such as luminal pressure or mucosal stroking, bacterial toxins (e.g., cholera toxin), and cytotoxic drugs which nonspecifically damage the cells, (e.g., cisplatinum) (2).
There is also classical receptor mediated stimulation via β-adrenergic, purinergic A2A/B and muscarinic receptors, together with inhibitory α2-adrenergic, histamine type 3 receptors and purinergic A1 receptors. These probably act through modulating intracellular Ca++, a surge in which is associated with 5-HT release (1).
Serotonin can stimulate 5-HT3 receptors with resultant inhibit gastric secretions, and stimulate ions of migrating motor complex (MMCs) (3) (with enhancement of intestinal secretions thereby accelerating small bowel transit) (4). They also stimulate antral contractions and vagal afferents inducing nausea (3).
5-HT3 antagonists inhibit splanchnic afferent nerve response to painful distension and inhibit vagal responses to chemotherapy induced 5-HT release. They also inhibit discharge of secreto-motor nerves, which act via VIP, and NO (1).
It is known that certain chemotherapy agents release large amounts of serotonin from the enterochromaffin cells in the gut. Serotonin acts on 5-HT3 receptors in the gut and brain stem. The vagal afferent nerves, the solitary tract nucleus (STN) and the area postrema (chemoreceptor trigger zone) are critical sites where 5-HT3 receptors are found. When patients are given chemotherapy agents, serotonin is released by the enterochromaffin cells in the small intestine stimulating vagal afferent nerves (via the 5-HT3 receptors) and this action initiates the vomiting reflex. The 5-HT3 receptor antagonists suppress nausea and vomiting by inhibiting serotonin from binding to the 5-HT3 receptors. The highest concentration of 5-HT3 receptors are found in the solitary tract nucleus (STN) and the chemoreceptor trigger zone (CTZ) of the central nervous system. It is believed that the 5-HT3 receptor antagonists suppress nausea and vomiting at the STN and CTZ sites. The 5-HT3 receptor antagonists prevent serotonin from activating and sensitizing the vagal afferent nerves which causes nausea and vomiting.
5-HT3 receptors
All the 5-HT receptors are G receptor protein-coupled receptors except for the 5-HT3 receptor that belongs to the Cys-loop superfamily of ligand-gated in ion channels (5). The activating inward current is cation-selective which is largely with sodium and potassium ions (5). The 5-HT3 receptor is made up of five subunits (encoded by the genes HTR3A, ATR3B, HTA3a, HTR3D, and/or HTR3E) arranged around a central ion conducting pore. A functional channel may be composed of either five identical 5-HT3A subunits (homopentameric) or a mixture of 5-HT3A and one of the other four receptor subunits (5-HT3B, 5-HT3C, 5-HT3D, 5-HT3E) (heteropentameric) (6).
Granisetron, ondansetron and palonosetron have slightly different receptor specificity. Palonosetron is a highly selective, high affinity competitive antagonist of the 5-HT3A receptor, whereas granisetron is highly specific for all subtypes of 5-HT3 receptors but has little or no affinity for 5-HT1, 5-HT2 and 5-HT4 receptors. Ondansetron also binds to the 5-HT1B, 5-HT1C, α1 adrenergic and muopioid receptors (Table 1). The clinical relevance of these findings is not clear (7).
Full table
5-HT3 receptor amtagonists
The chemical structures of the first generation 5-HT3 receptor amtagonists have been categorized into three major classes (7): (I) Carbazole derivatives (ondansetron); (II) Indazoles (granisetron); and (III) Indoles (dolasetron). Palonosetron is a highly selective second generation 5-HT3 receptor antagonist that has two stereogenic centers and may exist as four steroisomers (8). Palonosetron has a longer half-life (40 h) and greater receptor binding affinity (>30 fold) versus first generation 5-HT3RAs.
The second generation palonoestron has a much longer half life than the first generation 5-HT3 antagonists and a greater than 30 fold 5-HT3 receptor binding affinity. Although all the 5-HT3 antagonists share much of the same mechanisms of action, they have different chemical structures, binding affinities, dose responses and duration of effects. They are metabolized differently as different components of the cytochrome P450 system are predominate in the metabolism of the antagonists.
A correlation exists between the number of active CYP 2D6 alleles and number of vomiting episodes a patient may have, that is, the more active alleles a patient has the more likely they will be unresponsive to the antiemetic and vice versa.
5-HT3 receptor antagonists are often used in conjunction with glucocorticoid steroids such as dexamethasone in chemotherapy induced nausea and vomiting. Some studies advocate for intravenously administered 5-HT3 antagonists, others argue for oral administration. The concomitant use of aprepitant, an NK1 receptor antagonist, significantly increases the efficacy of the 5-HT3 antagonist in acute or delayed chemotherapy induced nausea and vomiting.
In a study which was a meta analysis of randomized control trials comparing 5-HT3 receptor antagonists and non-5-HT3 antagonists in post-op breast surgery patients, the 5-HT3 antagonists were found superior to placebo or active controls in the prevention of post-op nausea and vomiting (9). 5-HT3 antagonists were also superior to placebo in preventing nausea alone and were effective in reducing post op vomiting and the use of rescue antiemetics. 5-HT3 antagonists did not cause a significantly higher incidence of adverse events as compared to placebo.
Several studies comparing ganisetron alone versus in combination with dexamethasone or droperidol found that the combination showed greater efficacy than ganisetron alone in post op nausea and vomiting (10-12). However, in the absence of large randomized trials, no single agent has emerged as the standard of care for preventing post op nausea and vomiting in women undergoing breast surgery (9).
5-HT3RA agents
The intravenous administration of 5-HT3 receptor antagonists is followed by rapid distribution within the body. Dolasetron is rapidly eliminated (t1/2β <10 min) and metabolized to the active form, hydrodolasetron. A ubiquitous enzyme, carbonyl reductase, mediates the reduction of dolasetron to hydrodolasetron (13). Hydrodolasetron is 70% bound to plasma protein and has a t1/2β of approximately 7 h. It is predominantly metabolized by cytochrome P450 (CYP) CYP2D6 in the liver. Fiftythree percent of an administered intravenous dose of dolasetron is excreted unchanged in the urine (13,14).
The recommended adult dosage for oral Granisetron (Kytril) is 2 mg once daily or 1 mg twice daily in emetogenic chemotherapy and radiation. In the 2-mg oncedaily regimen, 21-mg tablets or 10 mL of granisetron oral solution (2 teaspoonfuls, equivalent to 2 mg) is given 1 hour before chemotherapy. In the 1 mg twice-daily regimen, the first 1-mg tablet or 1 teaspoonful (5 mL) of granisetron oral solution is given 1 hour before chemotherapy, and the second tablet or second teaspoonful (5 mL) of oral solution, 12 hours after the first (15). As with ondansetron, QT prolongation has been reported with Granisetron (15). Agents such as ondansetron and dolasetron that block sodium channels may produce QRS widening, and blocking potassium channels may lead to QT prolongation. Ondansetron and dolasetron may prolong QT intervals by up to about 5% (16). Yavas and colleagues conducted a prospective study in efforts to determine acute effects of palonosetron on electrocardiographic (ECG) parameters in cancer patients (17). Although median QT min value was higher after palonosetron administration than before palonosetron administration, the difference was not statistically significant (P=0.6) (17).
Thus, if clinicians were concerned about initiating a 5-HT3RA in a patient with pre-existing cardiac issues, a QT interval near the upper limit of normal, and the risk of arrhythmogenic potential; then palonosetron may be the 5-HT3RA of choice coupled with cautious dosing, close and frequent clinical follow-up, and frequent electrocardiographic monitoring.
Granisetron is metabolized by the liver through N-demethylation, aromatic ring oxidation, followed by conjugation (18). The elimination half-life of granisetron is between 5 and 8 h. Unlike the other 5-HT3 receptor antagonists, granisetron’s major route of metabolism is mediated by the P450 CYP3A isoenzyme (14). Clearance is predominantly by hepatic metabolism. In normal volunteers, 11% of the oral dose is eliminated unchanged in urine, 48% as metabolites in urine, and 38% as metabolites in feces in 48 hours (15).
Ondansetron has a short t1/2β of approximately 3 to 5 h (19). Seventy to seventy-six percent of ondansetron is protein bound. It is extensively metabolized via CYP3A4 in the liver by the hydroxylation of the indole ring followed by glucuronide or sulfate conjugation (14,19).
Palonosetron has the longest elimination half-life of 40 h (Table 1) (20). It is 62% bound to plasma proteins (21). The liver metabolizes approximately 50% of palonosetron. The two primary metabolites, N-oxide-palonosetron and 6-(S)- hydroxypalonosetron, are essentially inactive (14,21).
5-HT3RA agents not available in the U.S include tropisetron, ramosetron, and for irritable bowel disease where diarrhea is the dominant symptom---cilansetron and alsoetron (alosetron was withdrawn from U.S. market in 2000 due to adverse effects) [e.g., ischemic colitis], and is only available through a restrictive Risk Evaluation and Mitigation Strategies (REMS) program to patients meeting certain requirements.
It is conceivable that simultaneous multiple routes of administration of 5-HTRAs may be beneficial under certain circumstances. Ryu and colleagues conducted a prospective, randomized, double-blind study was to compare the efficacy and tolerability of intravenous, oral, and the combination of oral and intravenous ramosetron for PONV prophylaxis in patients undergoing laparoscopic cholecystectomy (22). Patients scheduled for laparoscopic cholecystectomy were double-randomly allocated to 1 of 3 groups. Patients were randomly allocated to receive either 0.3 mg of intravenous ramosetron (group A), 0.1 mg of oral ramosetron (group B), or the combination of 0.1 mg of oral ramosetron and 0.3 mg of intravenous ramosetron (group C). All patients received standardized balanced anesthesia with desflurane and remifentanil. Postoperative nausea, retching, vomiting, pain, and adverse effects were assessed at 0 to 2, 2 to 24, and 24 to 48 hours after surgery. They found that the combination of 0.1-mg oral and 0.3-mg intravenous ramosetron was more effective than either 0.3-mg intravenous ramosetron or 0.1-mg oral ramosetron alone for the prophylaxis of nausea and vomiting after laparoscopic cholecystectomy during the first 24 hours after surgery (22).
Non-5-HT3RA agents may also have antagonist effects at 5-HT3 receptor. Metoclopramide possesses some weak antagonist effects at the 5-HT3R. Mirtazapine and olanzapine have significant 5-HT3RA properties. Galanolactone, a diterpenoid found in ginger, is a 5-HT3A and felt to be at least partially responsible for the antiemetic mechanisms of ginger (23).
Genetic polymorphism in the cytochrome P450 monooxygenase system, drug efflux transporter adenosine triphosphate-binding cassette subfamily B member 1 and 5-hydroxytryptamine type 3 receptor subunits with differences in 5-HT3RAs interacting with different 5-HT3 receptor subtypes, also contribute to the interindividual variation in response to different 5-hydroxytryptamine type 3 receptor antagonists (14).
Kaiser et al. showed that patients who were ultrarapid metabolizers experienced more vomiting compared with extensive or poor metabolizers when given ondansetron and tropisetron in the treatment of chemotherapy-induced nausea and vomiting (24). This difference was predictably more pronounced for tropisetron than ondansetron, because tropisetron was primarily dependent on the CYP2D6 isoenzyme for metabolism (14).
The adenosine triphosphate-binding cassette subfamily B member 1 (ABCB1) drug transporter (also known as P-glycoprotein or MDR-1) is a transmembrane efflux pump in many tissues including the blood-brain barrier (25). Patients who were homozygous for the ABCB1 3435T allele responded better to antiemetic therapy compared with individuals who were heterozygous or homozygous for the ABCB1 3435C allele. This difference reached statistical significance in the granisetron-treated group (26).
Summary
Nausea and vomiting is among the most distressing symptoms to many patients. The release of serotonin may occur in a variety of medical conditions/interventions. Serotonin may contribute to nausea and vomiting via binding to the 5-HT3 receptor and in those states where this is a predominant factor, 5-HT3 receptor antagonists (5-HT3RAs) may be particularly effective as antiemetic agents.
Acknowledgements
The author would like to thank Pya Seidner for her enormous assistance in the preparation of this manuscript. Disclosure: The authors have no disclosure and has not published or submitted this manuscript elsewhere.
References
- Spiller R. Serotonergic modulating drugs for functional gastrointestinal diseases. Br J Clin Pharmacol 2002;54:11-20.
- Schwörer H, Rack?K, Kilbinger H. Cisplatin increases the release of 5-hydroxytryptamine (5-HT) from the isolated vascularly perfused small intestine of the guinea-pig: involvement of 5-HT3 receptors. Naunyn Schmiedebergs Arch Pharmacol 1991;344:143-9.
- Coleman NS, Wright J, Parker M, et al. MKC-733, a selective 5-HT3 receptor agonist stimulates fasting human antral motility. Gastroenterology 2001;120:A460-1.
- Coleman NS, Marciani L, Blackshaw PE, et al. MKC- 733, a selective 5-HT3 receptor agonist stimulates small bowel transit and relaxes the gastric fundus in man. Gastroenterology 2001;120:A71.
- Barnes NM, Hales TG, Lummis SC, et al. The 5-HT3 receptor-the relationship between structure and function. Neuropharmacology 2009;56:273-84.
- Gan TJ. Selective serotonin 5-HT3 receptor antagonists for postoperative nausea and vomiting: are they all the same? CNS Drugs 2005;19:225-38.
- Ho KY, Gan TJ. Pharmacology, pharmacogenetics, and clinical efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Curr Opin Anaesthesiol 2006;19:606-11.
- Tian K, Chen H, Tang J, et al. Enantioseparation of palonosetron hydrochloride by micellar electrokinetic chromatography with sodium cholate as chiral selector. J Chromatogr A 2006;1132:333-6.
- Singhal AK, Kannan S, Gota VS. 5HT3 antagonists for prophylaxis of postoperative nausea and vomiting in breast surgery: a meta-analysis. J Postgrad Med 2012;58:23-31.
- Dua N, Bhatnagar S, Mishra S, et al. Granisetron and ondansetron for prevention of nausea and vomiting in patients undergoing modified radical mastectomy. Anaesth Intensive Care 2004;32:761-4.
- Gan TJ, Jiao KR, Zenn M, et al. A randomized controlled comparison of electro-acupoint stimulation or ondansetron versus placebo for the prevention of postoperative nausea and vomiting. Anesth Analg 2004;99:1070-5, table of contents.
- Reihnér E, Grunditz R, Giesecke K, et al. Postoperative nausea and vomiting after breast surgery: efficacy of prophylactic ondansetron and droperidol in a randomized placebo-controlled study. Eur J Anaesthesiol 2000;17:197-203.
- Anzemet (dolasetron mesylate) injection (prescribing information). Kansas City, MO: Aventis Pharmaceuticals Inc, 2005.
- Ho KY, Gan TJ. Pharmacology, pharmacogenetics, and clinical efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Curr Opin Anaesthesiol 2006;19:606-11.
- Hsu ES. A review of granisetron, 5-hydroxytryptamine3 receptor antagonists, and other antiemetics. Am J Ther 2010;17:476-86.
- Boike SC, Ilson B, Zariffa N, et al. Cardiovascular effects of i.v. granisetron at two administration rates and of ondansetron in healthy adults. Am J Health Syst Pharm 1997;54:1172-6.
- Yavas C, Dogan U, Yavas G, et al. Acute effect of palonosetron on electrocardiographic parameters in cancer patients: a prospective study. Support Care Cancer 2012;20:2343-7.
- Kytril (granisetron hydrochloride) injection (prescribing information). Nutley, NJ: Roche Pharmaceuticals, 2006.
- Zofran (ondansetron hydrochloride) injection premixed (prescribing information). Research Triangle Park, NC: GlaxoSmithKline, 2006.
- Aloxi (palonosetron hydrochloride) injection (prescribing information). Lugano, Switzerland: Helsinn Healthcare SA, 2006.
- Siddiqui MA, Scott LJ. Palonosetron. Drugs 2004;64:1125-32.
- Ryu JH, Jeon YT, Hwang JW, et al. Intravenous, oral, and the combination of intravenous and oral ramosetron for the prevention of nausea and vomiting after laparoscopic cholecystectomy: a randomized, double-blind, controlled trial. Clin Ther 2011;33:1162-72.
- Huang QR, Iwamoto M, Aoki S, et al. Anti-5- hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger. Chem Pharm Bull (Tokyo) 1991;39:397-9.
- Kaiser R, Sezer O, Papies A, et al. Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes. J Clin Oncol 2002;20:2805-11.
- Marzolini C, Paus E, Buclin T, et al. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther 2004;75:13-33.
- Babaoglu MO, Bayar B, Aynacioglu AS, et al. Association of the ABCB1 3435C>T polymorphism with antiemetic efficacy of 5-hydroxytryptamine type 3 antagonists. Clin Pharmacol Ther 2005;78:619-26.